ABSTRACT
Focal radiation therapy (RT) has attracted considerable attention as a combinatorial partner for immunotherapy (IT), largely reflecting a well-defined, predictable safety profile and at least some potential for immunostimulation. However, only a few RT-IT combinations have been tested successfully in patients with cancer, highlighting the urgent need for an improved understanding of the interaction between RT and IT in both preclinical and clinical scenarios. Every year since 2016, ImmunoRad gathers experts working at the interface between RT and IT to provide a forum for education and discussion, with the ultimate goal of fostering progress in the field at both preclinical and clinical levels. Here, we summarize the key concepts and findings presented at the Sixth Annual ImmunoRad conference.
KEYWORDS: dose and fractionation, FLASH radiotherapy, immune checkpoint inhibitors, immunomodulators, lymph node sparing, tumor-associated macrophages
Introduction
The landscape of cancer treatments has been revolutionized by the introduction of various immunotherapeutic agents, notably immune checkpoint inhibitors (ICIs).1,2 However, only a limited fraction of patients obtain long-term clinical benefit from immunotherapy (IT).3,4 In this context, radiotherapy (RT) has emerged as a promising tool to extend the therapeutic potential of IT.5–7 At least in some instances, RT can indeed elicit an “in situ vaccination” effect to jumpstart tumor-targeting immune responses that can be amplified with IT.8–10 However, RT can also mediate a variety of immunosuppressive effects11, and several obstacles remain against the widespread implementation of successful RT-IT combinations in the clinic.12,13
Since 2016, Weill Cornell Medicine (New York) and the Gustave Roussy Cancer Campus (Paris) have joined forces to organize an annual conference that provides a forum for education, discussion, and networking among investigators interested in developing safe and effective RT-IT combinations (ImmunoRad). ImmunoRad is alternated between New York and Paris, allowing for the participation of faculty and trainees working across the globe to promote worldwide networking and collaborations. Each year, ImmunoRad provides a unique opportunity to extend the assorted and interactive community of researchers working on RT-IT combinations, including early career as well as experienced scientists, representing a sparkling environment for sharing knowledge and accelerating research on this exciting field of study.
In 2022, Immunorad hosted 33 speakers coming from a variety of disciplines including cancer immunology, cell and molecular biology, computational biology, medical physics, immuno-oncology, and radiation oncology. These experts covered various aspects of basic and clinical research, providing an opportunity for vivid discussion over recent discoveries on resistance mechanisms and strategies to overcome them, predictive biomarker identification, patient management, and clinical trial design. The conference also included one Poster Session and a Continuing Medical Education (CME) activity in collaboration with the Society for Immunotherapy of Cancer (SITC). Here, following a rational order based on research topics, we summarize the key concepts and findings presented at the Sixth Annual ImmunoRad conference in September 2022 in New York City.
Core news
Radiotherapy and immunotherapy in preclinical models
Cancer therapy has achieved tremendous progress in the last decade, with ICIs deeply changing the treatment landscape of specific cancer types.1,2 The recognition that only a minority of patients with cancer benefit from ICI-based immunotherapy,3 however, has driven an intense wave of preclinical and clinical investigations aimed at identifying novel therapeutic partners for ICIs, including RT.
ATR serine/threonine kinase (ATR) is one of the principal kinases involved in the DNA damage response (DDR) to RT, and ATR inhibitors have been shown to sensitize cancer cells to chemotherapy and RT in preclinical tumor models14. Kevin Harrington (The Institute of Cancer Research, London, UK) presented his work showing that ATR inhibitors radiosensitize cancer cells by reducing homologous recombination and abrogating RT-induced cell cycle arrest in G2, an effect that is accompanied by the accumulation of interferogenic micronuclei. In line with this notion, ATR inhibitors combined with RT result in robust nucleic acid-dependent type I and II interferon (IFN) signaling, abundant secretion of chemokines involved in immune cell recruitment (i.e., CCL3, CCL5, and CXCL10) and hence superior T cell and natural killer (NK) cell-mediated anticancer immunity.15,16 Interestingly, such an NK cell response can be further boosted with ICIs targeting T cell immunoreceptor with Ig and ITIM domains (TIGIT) and programmed cell death 1 (PDCD1, best known as PD-1), at least in human papilloma virus (HPV)-negative murine oral squamous cell carcinomas.17 Jamie Honeychurch (University of Manchester, Manchester, UK) discussed a growing interest on the mechanisms through which RT might influence the interaction between NK and cancer cells. Published in vitro data from this team suggest indeed that RT can promote short-term resistance to immune effector molecules such as perforin 1 (PRF1), thus reducing (at least temporarily) cancer cell susceptibility to lysis by NK cells.18 In vivo models confirm that RT transiently decreases the cancer cell sensitivity to NK and T cell – mediated killing.18
Another point of considerable interest revolves around the possibility of using RT to convert “cold”, non-immunogenic tumors into “hot”, immunogenic lesions.19 In this setting, the effects of low-dose radiation therapy (LDRT, <2 Gy per fraction) remain largely unexplored. Early evidence presented by Fernanda G. Herrera (University of Lausanne, Switzerland) suggests that LDRT can reprogram the microenvironment of various mouse tumor models to mobilize innate and adaptive immune responses, ultimately engaging dendritic cells (DCs) and CD4+ effector T cells with cytolytic activity in support of tumor control.20,21 Moreover, Jim Welsh (MD Anderson Cancer Center, Houston, TX, USA) demonstrated that high-dose RT to primary mouse lung tumors combined with LDRT to secondary metastases plus systemic ICIs can effectively control metastatic tumors through the engagement of innate and adaptive immunity, a systemic response that has been dubbed “radscopal effect”.22,23
Sergio Quezada (University College London Cancer Institute, London, UK) showed that targeting interleukin 2 receptor subunit alpha (IL2RA, best known as CD25) with a monoclonal antibody (mAb) that enables antibody-dependent cell cytotoxicity (ADCC) and antibody-dependent cell phagocytosis (ADCP) but preserves interleukin 2 (IL2) signaling is a potent strategy to promote cancer rejection in mouse models of glioblastoma immunity.24 This effect reflects CD4+CD25+FOXP3+ regulatory T (TREG) cell depletion and consequent restoration of tumor-targeting immunity.24 Whether this strategy can be efficiently combined with RT remains to be investigated. Interestingly, Roberta Zappasodi (Weill Cornell Medicine, New York, NY, USA) showed that TREG cell immunosuppressive functions can be blocked with neoadjuvant cytotoxic T lymphocyte-associated protein 4 (CTLA4)-targeting ICIs in mouse models of glycolysis-defective mammary carcinoma, resulting in long-lasting tumor-specific immunological memory and protection from metastasis specifically in this tumor metabolic setting.25 These findings point to TREG cells and tumor metabolism as potential targets to investigate in the context of RT to limit immunosuppression in irradiated tumors.
From preclinical models to clinical translation
Considerable discussion revolved around the urgent need to significantly improve patient prognosis in several RT- and/or IT-resistant cancers. In this setting, Theodore Hong (Massachusetts General Hospital, Boston, MA, USA) presented the results of a single-arm, non-randomized phase II clinical trial combining RT (delivered in 3 fractions of 8 Gy each) with the PD-1 blocker nivolumab and the CTLA4 blocker ipilimumab in patients with microsatellite stable colorectal cancer (CRC) and pancreatic ductal adenocarcinoma (PDAC) (NCT03104439). Disease control rate was promising, and responding patients exhibited increased tumor infiltration by NK cells and signs of innate immune signaling in post-treatment biopsies,26 pointing to the successful engagement of anticancer immunity. In a different scenario (i.e., IT-sensitive microsatellite instable CRC), Nina Bhardwaj (Mount Sinai institute, New York, NY, USA) showed that tumors with a high load of frameshift mutations display significant infiltration by activated CD8+ memory T cells and superior clinical responses to PD-1 blockers.27 Whether RT can be harnessed to boost PD-1 sensitivity in patients with reduced amounts of frameshift mutations remains to be investigated.
Elizabeth Jaffee (Johns Hopkins University, Baltimore, MD, USA) presented several studies that are investigating the complex signaling networking between inflammatory and stromal cells that characterize the PDAC microenvironment, with the aim of converting PDAC into an immune-responsive tumor.28,29 Of note, the dismal disease outcome that is generally associated with PDAC often involves metastatic dissemination to the liver. In this context, Weiping Zou (University of Michigan, Ann Harbor, MI, USA) reported that liver metastases are resistant to IT because of the ability of liver-resident macrophages30,31 to promote the demise of tumor-targeting CD8+ T cells, pointing to a potential role for RT as a strategy to circumvent this immunosuppressive mechanism32.
Recent findings from a randomized clinical study enrolling locally advanced head and neck squamous cell carcinoma (HNSCC) failed to demonstrate an advantage for the addition of IT to standard-of-care chemoradiation,33 corroborating the existence of obstacles toward the successful clinical translation of RT-IT combinations. Charleen Chan (The Institute of Cancer Research, London, UK) presented data from a syngeneic mouse model of HPV+ HNSCC demonstrating that adjuvant PD-1 blockage started 7 days after RT improved tumor control as compared to other treatment schedules, which has important implications for clinical trial design. Along similar lines, Sana Karam (University of Colorado Cancer Center, Aurora, CO, USA) demonstrated that elective nodal irradiation (ENI) suppresses immune responses as potentially driven to tumor-targeting RT (delivered in 3 fractions of 8 Gy each) plus IT in mouse models of HNSCC, although it increases the risk for regional metastasis, globally pointing to tumor-targeting RT plus IT followed by delayed ENI or surgical node resection as to an optimal approach for the management of HNSCC.34 Importantly, similar findings have previously been reported in mouse CRC models by Ariel Marciscano (Weill Cornell Medicine, New York, NY, USA), who alluded to these results (based on a single RT fraction of 12 Gy) during his presentation.35 Irma Telarovic (University of Zurich, Zurich, Switzerland) presented additional data in support of this concept from her preclinical work in a mouse melanoma model (also based on a single RT fraction of 12 Gy).36
TCR signaling changes dynamically upon RT, indicating that there may be a specific therapeutic window for IT with PD-1 blockage37. Simon Knott (Cedars-Sinai Medical Center, Los Angeles, CA, USA) presented data from a window-of-opportunity clinical study investigating neoadjuvant PD-1 blockage followed by stereotactic body radiotherapy (SBRT) in women with resectable triple negative breast cancer (NCT03366844). This study involved the collection of a research biopsy shortly after PD−1 blockage, enabling the longitudinal dissection of tumor microenvironment (TME) alterations associated with pathological responses in the surgical piece. In the setting of relapsed/refractory large B-cell lymphoma, chimeric antigen receptor (CAR) T cells represent an effective treatment option.38 RT stands out as an advantageous partner for CAR T cells in various manners.39–41 First, focal RT can be used as a bridge therapy, while CAR T cells are manufactured (which takes multiple weeks).42 Moreover, as presented by Monica Guzman (Weill Cornell Medicine, New York, NY, USA) and Anna Mondino (IRCCS San Raffaele Scientific Institute, Milan, Italy), RT can be delivered to the entire mouse (in one fraction of 1 Gy) or locally (in 3 fractions of 8 Gy each) to extend the therapeutic potential of CAR T cells or TCR-engineered T cells in models of acute lymphoblastic leukemia (ALL)43,44 and prostate cancer (unpublished observations), respectively. Whether these observations relate to the ability of RT to promote the upregulation of death receptors (DRs) on the surface of malignant cells45 remains to be formally established.
Finally, Sean Pitroda (University of Chicago, Chicago, IL, USA) presented the first comprehensive immunogenomic analysis of a randomized Phase I clinical trial testing concurrent or sequential ablative RT plus dual PD−1/CTLA4 blockage as a first-line therapy in patients with non-small cell lung cancer (NSCLC).46 Importantly, concurrent IT was found to be superior to sequential IT at improving responses and OS in patients with immunologically cold, highly aneuploid tumors, but not in those with less aneuploid neoplasms.46 These observations not only confirm previous findings on the ability of ICIs to compensate for potential immunosuppressive effects of RT47,48 but also suggest that tumor aneuploidy may represent a potential biomarker to personalize the addition of RT to IT.
Immunomodulators and the TME
Considerable attention is currently being given to factors and mechanisms that may represent targets for immunostimulatory agents other than ICIs, both locally and systemically. In this setting, Stephen Shiao (Cedars-Sinai Medical Center, Los Angeles, CA, USA) presented original work on the regulation of tumor-targeting immune responses by intestinal fungi.49 Specifically, antifungal regimens were associated with improved immune tumor control by RT in mouse models of breast cancer and melanoma, whereas opposite results were obtained with antibacterial agents.49 Corroborating the potential relevance of these observations for cancer patients, high intratumoral levels of C-type lectin domain containing 7A (CLEC7A), a pattern recognition receptor activated by fungal components50, were negatively associated with survival in breast cancer patients.49 Further investigation is required to validate these findings in multiple tumor types. David Lyden (Weill Cornell Medicine, New York, NY, USA) discussed the role of extracellular vesicles (EVs) and notably exomeres as modulators of immunity as well as potential prognostic and therapeutic targets. Indeed, EVs (which are essentially secreted by all cell types) contain DNA, RNA, and proteins encapsulated in a lipid bilayer and can be transferred from cell to cell as a means of communication,51 for instance as metabolic regulators.52 Cancer cells secrete increased amounts of EVs upon interactions with other components of the TME.53 Of note, tissue- and plasma-derived EV proteins may serve as biomarkers for early oncogenesis, pre-metastatic niche formation, as well as organotropism during metastatic dissemination.54,55 Finally, Laura Santambrogio (Weill Cornell Medicine, New York, NY, USA) presented data regarding the biogenic amine 3-hydroxykynurenine (3-HKA), a metabolite produced by a lateral branch of the indoleamine 2,3-dioxygenase 1 (IDO1) pathway in DCs, lymphatic endothelial cells, and human cancer cell lines.56 3-HKA has been shown to mediate pronounced immunosuppressive effects in vivo, in a number of mouse models of autoimmune disorders including psoriasis and nephrotoxic nephritis.56 It will be interesting to determine whether 3-HKA can be efficiently targeted to improve the immunostimulatory effects of RT.
Ariel Marciscano (Weill Cornell Medicine, New York, NY, USA) discussed the promise of targeting the adenosine-signaling pathway as a potent inducer of intratumoral immunosuppression.57 Adenosine accumulates in the TME upon degradation of extracellular ATP by ectonucleotidases, including ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1, best known as CD39) and 5’-nucleotidase ecto (NT5E, best known as CD73).58 Based on promising results from preclinical models of breast carcinoma59 and CRC,60 CD73 blockers are currently tested in combination with RT in these oncological indications and in combination with anti-PD-L1 therapy in a randomized Phase 3 trial in lung cancer (NCT03875573). John Stagg (Université de Montréal, Montréal, Canada) provided additional insights into this pathway by discussing results that suggest that CD39 and CD73 have non-redundant cooperative functions in polarization of the TME, with unexpected links to the DNA damage response.61 Specifically, in mouse models of pancreatic carcinomas, CD73 appears to protect against DNA damage, correlating with preserved NAD levels and superior activity of the DNA repair protein poly(ADP-ribose) polymerase 1 (PARP1) and culminating with suppressed stimulator of interferon response cGAMP interactor 1 (STING1) signaling and quenched type I IFN responses.61 Sana Karam (University of Colorado Cancer Center, Aurora, CO, USA) presented additional results suggesting that immunosuppressive mechanisms other than PD-1 and CTLA4 signaling may provide novel targets to improve the therapeutic efficacy of RT, notably hepatitis A virus cellular receptor 2 (HAVCR2, best known as TIM−3) signaling, tumor necrosis factor receptor superfamily, member 9 (TNFRSF9; best known as 4-1BB or CD137) signaling, and TREG cell functions.62,63 Mary Helen Barcellos-Hoff (University of California San Francisco, San Francisco, CA, USA) added to these observations by discussing the therapeutic potential of targeting transforming growth factor beta (TGFβ). In a mouse model of glioblastoma and breast cancer brain metastases, radiation-induced TGFβ activity could be imaged by positron emission tomography in situ and inhibiting TGFβ in these models extended the survival benefits afforded by RT.64 These studies corroborate previous data demonstrating that TGFβ signaling opposes tumor-targeting immune responses driven by RT65–67 and cancer cell-intrinsic cytotoxicity of RT.68,69 The clinical relevance of these mechanisms is further supported by the ability of HPV to inhibit TGFβ signaling, which at least in part explains the superior sensitivity of HPV+ HNSCCs to RT and DNA-damaging chemotherapeutics as compared to their HPV− counterparts68. Silvia Formenti (Weill Cornell Medicine, New York, NY, USA) provided further clinical insights into the ability of RT to elicit tumor-targeting immune responses that can be successfully actioned with IT. Specifically, she shared her positive experience about combining SBRT with ipilimumab in patients with NSCLC, a setting in which clinical responses were associated (at least in some patients) with increased circulating type I IFN and the ability of RT of upregulating tumor-associated antigens (TAAs).70 Similar findings have been obtained by the same team in preclinical tumor models, which also highlighted a role for RT-driven DR upregulation of cancer cells as well as of cytotoxic CD4+ T cells in the efficacy of RT.71 Whether cytotoxic CD4+ T cells also participate in the clinical activity of RT remains to be formally elucidated. Along these lines, it will be important to decipher the role of normal tissue exposure in the efficacy of RT. Recent preclinical data in model of KRAS-driven lung cancer suggest indeed that normal club cells of the epithelial airways responding to RT secrete a factor, namely secretoglobin, family 1A, member 1 (SCGB1A1, also known as CC10) that support the therapeutic synergy between RT and ICIs.72
Anna Wilkins (The Institute of Cancer Research, London, UK) showed that cancer-associated fibroblasts (CAFs), a heterogeneous population of stromal cells that can mediate potent immunosuppressive effects,73 are associated with poor RT outcomes in rectal tumors,74 a detrimental effect that is paralleled by the establishment of fibrosis and can be prevented by dual TGFβ/PD-L1 blockage (at least in preclinical models of PDAC, glioblastoma, and lung carcinoma).66 On a similar note, Ralph Weichselbaum (University of Chicago, Chicago, IL, USA) presented preclinical findings demonstrating that targeting myeloid-derived suppressor cells (MDSCs), a population of immature myeloid cells with potent immunosuppressive activity that has been linked to poor RT outcomes in multiple preclinical tumor models,75 improves the efficacy of RT combined with STING1 agonists in mouse CRCs.76 Specifically, all-trans retinoic acid (ATRA) was found to promote myeloid cell differentiation toward a population of inflammatory TAMs that supported RT efficacy via activation of adaptive immune responses that could be boosted with PD-L1 inhibitors to favor abscopal responses.77 Overall, the mechanisms and diversity of immune alterations in irradiated tumors remain poorly understood, warranting more research aiming at the identification of clinically viable strategies to polarize the TME in support of successful RT-IT combinations.
Innovative approaches for radiation delivery
Technical progress achieved over the past two decades has enabled the development of innovative approaches for delivering RT to cancer patients. Whether these strategies may offer advantages over conventional RT techniques for the development of successful RT-IT combinations remains to be formally elucidated. Zachary Morris (University of Wisconsin, Madison, WI, USA) presented data demonstrating that targeted radionuclide therapy (TRT) – consisting in the delivery of a tumor-targeted radioisotope (e.g., 90Y-NM600)78 – can elicit anticancer immunity in preclinical models of cold tumors, an effect that depends on STING1 signaling and can be boosted not only with ICIs but also with non-ablative external beam RT at a single disease site.79 This promising approach combines systemic immunostimulation by TRT with an in situ vaccination strategy80,81 and has been proven feasible in a veterinary trial enrolling dogs with advanced-stage melanoma or osteosarcoma.82
Marie-Catherine Vozenin (Lausanne University Hospital, Lausanne, Switzerland) presented immunobiological aspects of ultra-high dose rate (FLASH) irradiation.83,84 Specifically, she discussed the superior ability of FLASH to spare normal tissues as compared to conventional RT, while preserving an equivalent efficacy against the tumor, an effect that appears to be independent from the organ-specific TME and the activation of anticancer immunity, but may involve differential lipid peroxidation and Fenton reactions.85 In line with this possibility, hypoxic cancer cells are more sensitive to transcriptional changes elicited by FLASH than their normoxic counterparts. In line with these observations, Lorea Iturri (Institut Curie, Orsay, France) showed that proton FLASH is comparable to conventional-rate proton irradiation at recruiting lymphoid cells to the TME of mouse glioblastoma but enables superior preservation of memory functions.86 Moreover, she presented data on the ability of minibeam RT (MBRT) – an innovative technique that involved spatial-dose modulation – to control rat glioblastomas upon the activation of anticancer immunity, an effect that was not parallel by elevated toxicity as in the case of conventional RT at an equivalent dose (30 Gy)87. Constantinos Koumenis (University of Pennsylvania, Philadelphia, PA, USA) presented findings corroborating the superior ability of proton FLASH compared to standard proton radiation to better spare intestinal function, including proliferation of epithelial cells, and reduce fibrosis while being equipotent in controlling PDAC growth in preclinical mouse models88. Early studies based on single-cell transcriptomics support a differential activation of the IFN response in the epithelial and immune compartments of the intestine exposed to proton FLASH vs. standard proton radiation, which may contribute to such a sparing effect. Robert Timmerman (UT Southwestern University, Dallas, TX, USA) introduced PULSAR (Personalized Ultrafractionated Stereotactic Adaptive Radiotherapy). PULSAR enables large intervals (weeks or months) between each RT dose by delivering high doses per faction, hence improving the tolerance of organs at risk and facilitating adaptations of treatment regimen based on tumor response and modification of its microenvironment.89 Specifically, PULSAR combined with a PD-L1 blocker was shown to mediate robust therapeutic effects in immunocompetent mouse models of CRC and lung carcinoma, an effect that was abrogated by CD8+ T cell depletion.90
Personalization of RT-IT strategies with imaging
One of the key issues for the development of successful RT-IT combinations is the lack of specific biomarkers that would predict the likelihood of individual patients to respond beyond standard parameters that are normally used to inform the usage of RT or IT as individual agents91. As discussed by Eric Deutsch (Gustave Roussy Cancer Center, Paris, France), one promising way to identify biomarkers to personalize treatment in the RT-IT setting is radiomics, a technique that allows investigators to extract quantitative information by medical imaging and to apply artificial intelligence for the discovery of predictive models of response92. Radiomics has indeed been successfully applied to develop an imaging biomarker of tumor-infiltrating CD8+ T cells in patients receiving IT93,94. Another field of recent development is the possibility to study the movement of lymphocytes in vivo within TDLNs via high-resolution three-photon microscopy. Chris Xu (Cornell University, Ithaca, NY, USA) presented work with three-photon microscopy visualizing CD4+ and CD8+ T cell motility in mouse lymph nodes. Specifically, CD4+ and CD8+ T cell distributions were found to be strongly related to antigen presenting with a critical role for local chemokine gradients.95 Whether these findings can be extrapolated to human lymph nodes and whether they may provide predictive information on the likelihood of individual patients to benefit from RT-IT combinations remain to be investigated.
Concluding remarks
Despite considerable progress at least in some oncological indications,70,96,97 several obstacles remain against the clinical implementation of successful RT-IT combinations across a wide range of oncological indications.13 Specifically, additional work is required to dissect the impact of dose and fractionation on the immunogenicity of RT, delineate approaches that limit the exposure of circulating lymphocytes and TDLNs (at least initially), define optimal treatment schedules for RT to synergize with IT (which may depend on tumor type and specific IT), characterize the potential benefits from low-dose exposure of normal tissues, and clarify the immunogenic potential of charged particles including protons (Figure 1). We are positive that progress in these directions will be accelerated by the framework provided by ImmunoRad, and we look forward to discussing the most recent discoveries as well as persisting challenges in the field at the Seventh ImmunoRad conference, which will be held in Paris in September 2023.
Figure 1.
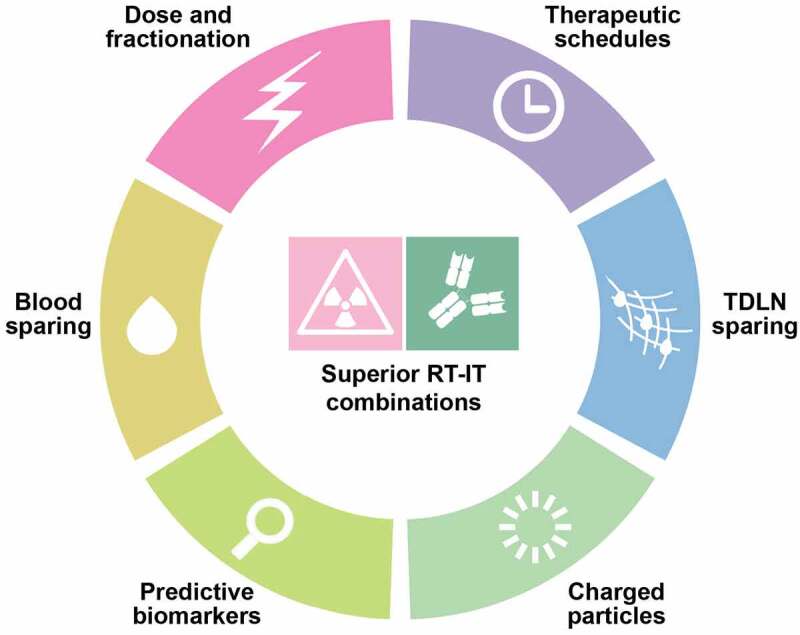
Persisting challenges for radiotherapy and immunotherapy combinations. We surmise that the successful implementation of radiotherapy (RT) and immunotherapy (IT) combinations to a wide spectrum of oncological indications will require an improved understanding of the impact of dose and fractionation on the immunogenicity or RT, the design of treatment fields that spare circulating lymphocytes and tumor-draining lymph nodes (TDLNs), at least initially, the identification of optimal treatment schedules to maximize the interaction between RT and IT (which may depend on tumor type and specific immunotherapeutic agent) and an advanced characterization of the immunobiological effects of charged particles.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Declaration of interest
MHBH is or has have been a recipient of research grants paid to UCSF or in-kind resources from Roche-Genentech, Varian Medical Systems, Eli Lilly, Pathway Innovations and has received fees for consulting from EMD-Serono, Varian Medical Systems, Genentech, Pathway Innovation, Scholar Rock. KHhas Honoraria: Arch Oncology (Inst), AstraZeneca (Inst), BMS (Inst), Boehringer Ingelheim (Inst), Codiak Biosciences (Inst), F-Star Therapeutics (Inst), Inzen Therapeutics (Inst), Merck Serono (Inst), MSD (Inst), Oncolys Biopharma (Inst), Pfizer (Inst), Replimune (Inst), VacV Biotherapeutics (Inst); Consulting or Advisory Role: Arch Oncology (Inst), AstraZeneca (Inst), BMS (Inst), Boehringer Ingelheim (Inst), Inzen Therapeutics (Inst), Merck Serono (Inst), MSD (Inst), Oncolys BioPharma (Inst), Replimune (Inst); Speakers’ Bureau: BMS (Inst), Merck Serono (Inst), MSD (Inst); Research Funding: AstraZeneca (Inst), Boehringer Ingelheim (Inst), Merck Sharp & Dohme (Inst), Replimune (Inst). FGH received Grant or Research Support Companies from Accuray inc, Bioprotect, Bristol-Myers Squibb, Roche-ImFlame/ImCore, Nanobiotix, AstraZeneca, Debio Pharmaceuticals, Seagen, Eisai, MSD; Grant or Research Support Foundations from Prostate Cancer Foundation, San Salvatore Foundation; Investigator or Co-Investigator Clinical Trials in Bristol-Myers Squibb; Consultations: Johnson & Johnson; Academic Collaborations: EORTC chairman Gynecology Cancer Group, ESMO Scientific Committee member for drug development, ASTRO Scientific Committee Annual Meeting. TH has Consulting: Synthetic Biologics, Novocure, Boston Scientific, Inivata, Merck, GSK; Scientific Advisory Board: PanTher Therapeutics (Equity), Lustgarten; Research Funding (Clinical Trials): Taiho, AstraZeneca, BMS, GSK, IntraOp, Ipsen. EJ reports other support from Abmeta and Adventris, personal fees from Achilles, Dragonfly, Mestag, The Medical Home Group, and Surgtx, other support from Parker Institute, grants and other support from the Lustgarten Foundation, Genentech, BMS, and Break Through Cancer outside the submitted work. SDK receives clinical funding from AstraZeneca, Genentech, and Ionis; she also receives preclinical research funding from Roche. KS is founder and consultant for Faeth Therapeutics and Transomic Technologies. CK is the co-recipient of a Sponsored Research Agreement from Ion Beam Applications (IBA). AM is funded by the Associazione Italiana per la Ricerca sul Cancro (AIRC IG 2018 Id.21763 and AIRC Programma di ricerca 5 per Mille 2019 Id.22737). MM declare grants from Boehringer Ingelheim, AC Biosciences and MSD outside the submitted work. ZSM has Scientific Advisory Board roles and equity options with Archeus Technologies and Seneca Therapeutics. JS owns stock and is a member of the Scientific Advisory Board of Surface Oncology, and is a member of the Scientific Advisory Board of Domain Therapeutics. RT has research grants to his institution from: Varian Medical Systems, Elekta Oncology, Accuray, Inc; scientific advisory board member for: Reflexion Medical, ImmuneSensor Therapeutics. AW acknowledge funding from AstraZeneca and imCORE. RW has stock and other ownership interests with Boost Therapeutics, Immvira LLC, Reflexion Pharmaceuticals, Coordination Pharmaceuticals Inc., Magi Therapeutics, Oncosenescence, Aqualung Therapeutics Corporation, and Cyntegron; he has served in a consulting or advisory role for Aettis Inc., AstraZeneca, Coordination Pharmaceuticals, Genus, Merck Serono S.A., Nano Proteagen, NKGen Biotech, Shuttle Pharmaceuticals, Highlight Therapeutics, S.L., Aqualung Therapeutics Corporation; he has research grants with Varian and Regeneron. RZ is scientific advisory board member of iTeos Therapeutics, receives research grant support from Bristol Myers Squibb and AstraZeneca, and is inventor on patent applications related to work on GITR, CTLA-4, and PD-1 (patent numbers: US20180244793A1; US10323091B2; WO2018106864A1; WO2019094352A1). SD has received compensation for consultant/advisory services from Lytix Biopharma, Mersana Therapeutics, EMD Serono, Ono Pharmaceutical, and Genentech, and research support from Lytix Biopharma and Boehringer-Ingelheim for unrelated projects. LG is/has been holding research contracts with Lytix Biopharma, Promontory and Onxeo, has received consulting/advisory honoraria from Boehringer Ingelheim, AstraZeneca, OmniSEQ, Onxeo, The Longevity Labs, Inzen, Imvax, Sotio, Promontory, Noxopharm, EduCom, and the Luke Heller TECPR2 Foundation, and holds Promontory stock options. ED reports grants and personal fees from Roche Genentech; grants from Servier; grants from AstraZeneca; grants and personal fees from Merck-Serono; grants from BMS; and grants from MSD outside the submitted work. SCF has Consultant: Bayer, Bristol Myers Squibb, Varian, ViewRay, Accuray, Elekta, Janssen, Regeneron, GlaxoSmithKline, Eisai, Astra Zeneca, MedImmune, Merck US, EMD Serono/Merck, Genentech/ROCHE, Boehringer Ingelheim, Nanobiotix and Grant/Research: support from: Bristol Myers Squibb, Varian, Regeneron, Merck, Celldex. All other authors have no conflict of interest to declare.
References
- 1.Sharma P, Allison JP.. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–10. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. 2022;21(7):509–528. doi: 10.1038/s41573-021-00345-8. [DOI] [PubMed] [Google Scholar]
- 3.Galluzzi L, Chan TA, Kroemer G, Wolchok JD, López-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018;10(459). doi: 10.1126/scitranslmed.aat7807. [DOI] [PubMed] [Google Scholar]
- 4.Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20(11):662–680. doi: 10.1038/s41568-020-0285-7. [DOI] [PubMed] [Google Scholar]
- 5.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105(4):256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutsch E, Chargari C, Galluzzi L, Kroemer G. Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol. 2019;20(8):e452–e463. doi: 10.1016/S1470-2045(19)30171-8. [DOI] [PubMed] [Google Scholar]
- 7.Formenti SC, Demaria S. Future of radiation and immunotherapy. Int J Radiat Oncol. 2020;108(1):3–5. doi: 10.1016/j.ijrobp.2020.04.034. [DOI] [PubMed] [Google Scholar]
- 8.Golden EB, Marciscano AE, Formenti SC. Radiation therapy and the in situ vaccination approach. Int J Radiat Oncol Biol Phys. 2020;108(4):891–898. doi: 10.1016/j.ijrobp.2020.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys. 2012;84(4):879–880. doi: 10.1016/j.ijrobp.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Ruiz ME, Vitale I, Harrington KJ, Melero I, Galluzzi L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat Immunol. 2020;21(2):120–134. doi: 10.1038/s41590-019-0561-4. [DOI] [PubMed] [Google Scholar]
- 11.Cytlak UM, Dyer DP, Honeychurch J, Williams KJ, Travis MA, Illidge TM. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat Rev Immunol. 2022;22(2):124–138. doi: 10.1038/s41577-021-00568-1. [DOI] [PubMed] [Google Scholar]
- 12.Hwang WL, Pike LRG, Royce TJ, Mahal BA, Loeffler JS. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol. 2018;15(8):477–494. doi: 10.1038/s41571-018-0046-7. [DOI] [PubMed] [Google Scholar]
- 13.Galluzzi L, Aryankalayil MJ, Coleman CN, Formenti SC. Emerging evidence for adapting radiotherapy to immunotherapy. Nat Rev Clin Oncol. 2023. doi: 10.1038/s41571-023-00782-x. [DOI] [PubMed] [Google Scholar]
- 14.Klapp V, Álvarez-Abril B, Leuzzi G, Kroemer G, Ciccia A, Galluzzi L. The DNA damage response and inflammation in cancer. Cancer Discov. 2023;OF1–OF25. IN PRESS. doi: 10.1158/2159-8290.CD-22-1220. [DOI] [PubMed] [Google Scholar]
- 15.Dillon MT, Barker HE, Pedersen M, Hafsi H, Bhide SA, Newbold KL, Nutting CM, McLaughlin M, Harrington KJ. Radiosensitization by the ATR Inhibitor AZD6738 through generation of acentric micronuclei. Mol Cancer Ther. 2017;16(1):25–34. doi: 10.1158/1535-7163.MCT-16-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dillon MT, Bergerhoff KF, Pedersen M, Whittock H, Crespo-Rodriguez E, Patin EC, Pearson A, Smith HG, Paget JTE, Patel RR, et al. ATR inhibition potentiates the radiation-induced inflammatory tumor microenvironment. Clin Cancer Res. 2019;25(11):3392–3403. doi: 10.1158/1078-0432.CCR-18-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patin EC, Dillon MT, Nenclares P, Grove L, Soliman H, Leslie I, Northcote D, Bozhanova G, Crespo-Rodriguez E, Baldock H, et al. Harnessing radiotherapy-induced NK-cell activity by combining DNA damage–response inhibition and immune checkpoint blockade. J ImmunoTher Cancer. 2022;10(3):e004306. doi: 10.1136/jitc-2021-004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuomela K, Mukherjee D, Ambrose AR, Harikrishnan A, Mole H, Hurlstone A, Önfelt B, Honeychurch J, Davis DM. Radiotherapy transiently reduces the sensitivity of cancer cells to lymphocyte cytotoxicity. Proc Natl Acad Sci U S A. 2022;119(3). doi: 10.1073/pnas.2111900119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demaria S, Coleman CN, Formenti SC. Radiotherapy: Changing the game in immunotherapy. Trends in Cancer. 2016;2(6):286–294. doi: 10.1016/j.trecan.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera FG, Ronet C, Ochoa de Olza M, Barras D, Crespo I, Andreatta M, Corria-Osorio J, Spill A, Benedetti F, Genolet R, et al. Low-Dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov. 2022;12(1):108–133. doi: 10.1158/2159-8290.CD-21-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao L, Zhang S, Kepp O, Kroemer G, Liu P. Dendritic cell transfer for cancer immunotherapy. Int Rev Cell Mol Biol. 2022;370:33–64. [DOI] [PubMed] [Google Scholar]
- 22.Barsoumian HB, Ramapriyan R, Younes AI, Caetano MS, Menon H, Comeaux NI, Cushman TR, Schoenhals JE, Cadena AP, Reilly TP, et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J ImmunoTher Cancer. 2020;8(2):e000537. doi: 10.1136/jitc-2020-000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel RR, He K, Barsoumian HB, Chang JY, Tang C, Verma V, Comeaux N, Chun SG, Gandhi S, Truong MT, et al. High-dose irradiation in combination with non-ablative low-dose radiation to treat metastatic disease after progression on immunotherapy: Results of a phase II trial. Radiother Oncol. 2021;162:60–67. doi: 10.1016/j.radonc.2021.06.037. [DOI] [PubMed] [Google Scholar]
- 24.Solomon I, Amann M, Goubier A, Arce Vargas F, Zervas D, Qing C, Henry JY, Ghorani E, Akarca AU, Marafioti T, et al. CD25-T(reg)-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity. Nat Cancer. 2020;1(12):1153–1166. doi: 10.1038/s43018-020-00133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zappasodi R, Serganova I, Cohen IJ, Maeda M, Shindo M, Senbabaoglu Y, Watson MJ, Leftin A, Maniyar R, Verma S, et al. CTLA-4 blockade drives loss of T(reg) stability in glycolysis-low tumours. Nature. 2021;591(7851):652–658. doi: 10.1038/s41586-021-03326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parikh AR, Szabolcs A, Allen JN, Clark JW, Wo JY, Raabe M, Thel H, Hoyos D, Mehta A, Arshad S, et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat Cancer. 2021;2(11):1124–1135. doi: 10.1038/s43018-021-00269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roudko V, Bozkus CC, Orfanelli T, McClain CB, Carr C, O’Donnell T, Chakraborty L, Samstein R, Huang K-L, Blank SV, et al. Shared immunogenic poly-epitope frameshift mutations in microsatellite unstable tumors. Cell. 2020;183(6):1634–1649.e1617. doi: 10.1016/j.cell.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popovic A, Jaffee EM, Zaidi N. Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J Clin Invest. 2018;128(8):3209–3218. doi: 10.1172/JCI120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gartrell RD, Enzler T, Kim PS, Fullerton BT, Fazlollahi L, Chen AX, Minns HE, Perni S, Weisberg SP, Rizk EM, et al. Neoadjuvant chemoradiation alters the immune microenvironment in pancreatic ductal adenocarcinoma. Oncoimmunology. 2022;11(1):2066767. doi: 10.1080/2162402X.2022.2066767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao X, Lai SWT, Chen S, Wang S, Feng M. Targeting tumor-associated macrophages for cancer immunotherapy. Int Rev Cell Mol Biol. 2022;368:61–108. [DOI] [PubMed] [Google Scholar]
- 31.Papachristoforou E, Ramachandran P. Macrophages as key regulators of liver health and disease. Int Rev Cell Mol Biol. 2022;368:143–212. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27(1):152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, Harrington K, Chang PMH, Lin J-C, Razaq MA, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22(4):450–462. doi: 10.1016/S1470-2045(20)30737-3. [DOI] [PubMed] [Google Scholar]
- 34.Darragh LB, Gadwa J, Pham TT, Van Court B, Neupert B, Olimpo NA, Nguyen K, Nguyen D, Knitz MW, Hoen M, et al. Elective nodal irradiation mitigates local and systemic immunity generated by combination radiation and immunotherapy in head and neck tumors. Nat Commun. 2022;13(1):7015. doi: 10.1038/s41467-022-34676-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marciscano AE, Ghasemzadeh A, Nirschl TR, Theodros D, Kochel CM, Francica BJ, Muroyama Y, Anders RA, Sharabi AB, Velarde E, et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin Cancer Res. 2018;24(20):5058–5071. doi: 10.1158/1078-0432.CCR-17-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Telarovic I, Yong CSM, Guckenberger M, Unkelbach J, Pruschy M. Radiation-induced lymphopenia does not impact treatment efficacy in a mouse tumor model. Neoplasia. 2022;31:100812. doi: 10.1016/j.neo.2022.100812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santa-Maria CA, Dunn SA, Ho AY. Immunotherapy combined with radiation therapy in breast cancer: a rapidly evolving landscape. Semin Radiat Oncol. 2022;32(3):291–297. doi: 10.1016/j.semradonc.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Roschewski M, Longo DL, Wilson WH. CAR T-Cell therapy for large B-Cell lymphoma — who, when, and how? N Engl J Med. 2022;386(7):692–696. doi: 10.1056/NEJMe2118899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonana-Vildosola A, Zanetti SR, Palazon A. Enabling CAR-T cells for solid tumors: rage against the suppressive tumor microenvironment. Int Rev Cell Mol Biol. 2022;370:123–147. [DOI] [PubMed] [Google Scholar]
- 40.Laurent PA, Morel D, Meziani L, Depil S, Deutsch E. Radiotherapy as a means to increase the efficacy of T-cell therapy in solid tumors. Oncoimmunology. 2023;12(1):2158013. doi: 10.1080/2162402X.2022.2158013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Navarro EA, et al. What will (and should) be improved in CAR immunotherapy? Int Rev Cell Mol Biol. 2022;370:149–161. doi: 10.1016/bs.ircmb.2022.04.002 [DOI] [PubMed] [Google Scholar]
- 42.Amini L, Silbert SK, Maude SL, Nastoupil LJ, Ramos CA, Brentjens RJ, Sauter CS, Shah NN, Abou-el-Enein M. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat Rev Clin Oncol. 2022;19(5):342–355. doi: 10.1038/s41571-022-00607-3. [DOI] [PubMed] [Google Scholar]
- 43.Yamazaki T, Sugita M, Martinet J, Boyer O, Galluzzi L, Guzman ML, Formenti SC. Boosting CAR T cell expansion and therapeutic activity with low-dose radiation therapy. Int J Radiat Oncol. 2020;108(3):S158–S159. doi: 10.1016/j.ijrobp.2020.07.920. [DOI] [Google Scholar]
- 44.Sugita M, Yamazaki T, Alhomoud M, Martinet J, Latouche J-B, Golden E, Boyer O, Van Besien K, Formenti SC, Galluzzi L, et al. Radiation therapy improves CAR T cell activity in acute lymphoblastic leukemia. Cell Death Disease. 2023;14(5):IN PRESS. doi: 10.1038/s41419-023-05829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeSelm C, Palomba ML, Yahalom J, Hamieh M, Eyquem J, Rajasekhar VK, Sadelain M. Low-Dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol Ther. 2018;26(11):2542–2552. doi: 10.1016/j.ymthe.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spurr LF, Martinez CA, Kang W, Chen M, Zha Y, Hseu R, Gutiontov SI, Turchan WT, Lynch CM, Pointer KB, et al. Highly aneuploid non-small cell lung cancer shows enhanced responsiveness to concurrent radiation and immune checkpoint blockade. Nat Cancer. 2022;3(12):1498–1512. doi: 10.1038/s43018-022-00467-x. [DOI] [PubMed] [Google Scholar]
- 47.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen HY, Xu L, Li L-F, Liu X-X, Gao J-X, Bai Y-R. Inhibiting the CD8(+) T cell infiltration in the tumor microenvironment after radiotherapy is an important mechanism of radioresistance. Sci Rep. 2018;8(1):11934. doi: 10.1038/s41598-018-30417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiao SL, Kershaw KM, Limon JJ, You S, Yoon J, Ko EY, Guarnerio J, Potdar AA, McGovern DPB, Bose S, et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell. 2021;39(9):1202–1213.e1206. doi: 10.1016/j.ccell.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Man SM, Jenkins BJ. Context-dependent functions of pattern recognition receptors in cancer. Nat Rev Cancer. 2022;22(7):397–413. doi: 10.1038/s41568-022-00462-5. [DOI] [PubMed] [Google Scholar]
- 51.Dixson AC, Dawson TR, Di Vizio D, Weaver AM. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat Rev Mol Cell Biol. 2023. doi: 10.1038/s41580-023-00576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang G, Zhang H, Lyden D. Tumour-regulated anorexia preceding cachexia. Nat Cell Biol. 2021;23(2):111–113. doi: 10.1038/s41556-021-00635-8. [DOI] [PubMed] [Google Scholar]
- 53.Choi J, Cho HY, Jeon J, Kim K-A, Han YD, Ahn JB, Wortzel I, Lyden D, Kim HS. Detection of circulating KRAS mutant DNA in extracellular vesicles using droplet digital PCR in patients with colon cancer. Front Oncol. 2022;12:1067210. doi: 10.3389/fonc.2022.1067210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, Zambirinis CP, Rodrigues G, Molina H, Heissel S, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182(4):1044–1061.e1018. doi: 10.1016/j.cell.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clement CC, D’Alessandro A, Thangaswamy S, Chalmers S, Furtado R, Spada S, Mondanelli G, Ianni F, Gehrke S, Gargaro M, et al. 3-hydroxy-L-kynurenamine is an immunomodulatory biogenic amine. Nat Commun. 2021;12(1):4447. doi: 10.1038/s41467-021-24785-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allard B, Allard D, Buisseret L, Stagg J. The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol. 2020;17(10):611–629. doi: 10.1038/s41571-020-0382-2. [DOI] [PubMed] [Google Scholar]
- 58.Kepp O, Bezu L, Yamazaki T, Di Virgilio F, Smyth MJ, Kroemer G, Galluzzi L. ATP and cancer immunosurveillance. Embo J. 2021;40(13):e108130. doi: 10.15252/embj.2021108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wennerberg E, Spada S, Rudqvist N-P, Lhuillier C, Gruber S, Chen Q, Zhang F, Zhou XK, Gross SS, Formenti SC, et al. CD73 blockade promotes dendritic cell infiltration of irradiated tumors and tumor rejection. Cancer Immunol Res. 2020;8(4):465–478. doi: 10.1158/2326-6066.CIR-19-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsukui H, Horie H, Koinuma K, Ohzawa H, Sakuma Y, Hosoya Y, Yamaguchi H, Yoshimura K, Lefor AK, Sata N, et al. CD73 blockade enhances the local and abscopal effects of radiotherapy in a murine rectal cancer model. Bmc Cancer. 2020;20(1):411. doi: 10.1186/s12885-020-06893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacoberger-Foissac C, Cousineau I, Bareche Y, Allard D, Chrobak P, Allard B, Pommey S, Messaoudi N, McNicoll Y, Soucy G, et al. CD73 inhibits cgas–sting and cooperates with cd39 to promote pancreatic cancer. Cancer Immunol Res. 2023;11(1):56–71. doi: 10.1158/2326-6066.CIR-22-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oweida A, Hararah MK, Phan A, Binder D, Bhatia S, Lennon S, Bukkapatnam S, Van Court B, Uyanga N, Darragh L, et al. Resistance to Radiotherapy and PD-L1 Blockade is Mediated by TIM-3 Upregulation and Regulatory T-Cell Infiltration. Clin Cancer Res. 2018;24(21):5368–5380. doi: 10.1158/1078-0432.CCR-18-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knitz MW, Bickett TE, Darragh LB, Oweida AJ, Bhatia S, Van Court B, Bhuvane S, Piper M, Gadwa J, Mueller AC, et al. Targeting resistance to radiation-immunotherapy in cold HNSCCs by modulating the Treg-dendritic cell axis. J ImmunoTher Cancer. 2021;9(4):e001955. doi: 10.1136/jitc-2020-001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez-Junca A, Reiners O, Borrero-Garcia LD, Beckford-Vera D, Lazar AA, Chou W, Braunstein S, VanBrocklin H, Franc BL, Barcellos-Hoff MH. Positron Emission Tomography Imaging of Functional Transforming Growth Factor β (TGFβ) Activity and Benefit of TGFβ Inhibition in Irradiated Intracranial Tumors. Int J Radiat Oncol Biol Phys. 2021;109(2):527–539. doi: 10.1016/j.ijrobp.2020.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Formenti SC, Lee P, Adams S, Goldberg JD, Li X, Xie MW, Ratikan JA, Felix C, Hwang L, Faull KF, et al. Focal Irradiation and Systemic TGFβ Blockade in Metastatic Breast Cancer. Clin Cancer Res. 2018;24(11):2493–2504. doi: 10.1158/1078-0432.CCR-17-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lan Y, Moustafa M, Knoll M, Xu C, Furkel J, Lazorchak A, Yeung T-L, Hasheminasab S-M, Jenkins MH, Meister S, et al. Simultaneous targeting of TGF-β/PD-L1 synergizes with radiotherapy by reprogramming the tumor microenvironment to overcome immune evasion. Cancer Cell. 2021;39(10):1388–1403.e1310. doi: 10.1016/j.ccell.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez-Ruiz ME, Rodríguez I, Mayorga L, Labiano T, Barbes B, Etxeberria I, Ponz-Sarvise M, Azpilikueta A, Bolaños E, Sanmamed MF, et al. TGFβ Blockade Enhances Radiotherapy Abscopal Efficacy Effects in Combination with Anti-PD1 and Anti-CD137 Immunostimulatory Monoclonal Antibodies. Mol Cancer Ther. 2019;18(3):621–631. doi: 10.1158/1535-7163.MCT-18-0558. [DOI] [PubMed] [Google Scholar]
- 68.Liu Q, Palomero L, Moore J, Guix I, Espín R, Aytés A, Mao J-H, Paulovich AG, Whiteaker JR, Ivey RG, et al. Loss of TGFβ signaling increases alternative end-joining DNA repair that sensitizes to genotoxic therapies across cancer types. Sci Transl Med. 2021;13(580). doi: 10.1126/scitranslmed.abc4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Q, Ma L, Jones T, Palomero L, Pujana MA, Martinez-Ruiz H, Ha PK, Murnane J, Cuartas I, Seoane J, et al. Subjugation of TGFβ Signaling by Human Papilloma Virus in Head and Neck Squamous Cell Carcinoma Shifts DNA Repair from Homologous Recombination to Alternative End Joining. Clin Cancer Res. 2018;24(23):6001–6014. doi: 10.1158/1078-0432.CCR-18-1346. [DOI] [PubMed] [Google Scholar]
- 70.Formenti SC, Rudqvist N-P, Golden E, Cooper B, Wennerberg E, Lhuillier C, Vanpouille-Box C, Friedman K, Ferrari de Andrade L, Wucherpfennig KW, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24(12):1845–1851. doi: 10.1038/s41591-018-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lhuillier C, Rudqvist N-P, Yamazaki T, Zhang T, Charpentier M, Galluzzi L, Dephoure N, Clement CC, Santambrogio L, Zhou XK, et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J Clin Invest. 2021;131(5). doi: 10.1172/JCI138740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ban Y, Markowitz GJ, Zou Y, Ramchandani D, Kraynak J, Sheng J, Lee SB, Wong STC, Altorki NK, Gao D, et al. Radiation-activated secretory proteins of Scgb1a1(+) club cells increase the efficacy of immune checkpoint blockade in lung cancer. Nat Cancer. 2021;2(9):919–931. doi: 10.1038/s43018-021-00245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L, Bernard C, et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell. 2018;33(3):463–479.e410. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 74.Wilkins A, Fontana E, Nyamundanda G, Ragulan C, Patil Y, Mansfield D, Kingston J, Errington-Mais F, Bottomley D, von Loga K, et al. Differential and longitudinal immune gene patterns associated with reprogrammed microenvironment and viral mimicry in response to neoadjuvant radiotherapy in rectal cancer. J ImmunoTher Cancer. 2021;9(3):e001717. doi: 10.1136/jitc-2020-001717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jimenez-Cortegana C, Galassi C, Klapp V, Gabrilovich DI, Galluzzi L. Myeloid-Derived Suppressor Cells and Radiotherapy. Cancer Immunol Res. 2022;10(5):545–557. doi: 10.1158/2326-6066.CIR-21-1105. [DOI] [PubMed] [Google Scholar]
- 76.Liang H, Deng L, Hou Y, Meng X, Huang X, Rao E, Zheng W, Mauceri H, Mack M, Xu M, et al. Host STING-dependent MDSC mobilization drives extrinsic radiation resistance. Nat Commun. 2017;8(1):1736. doi: 10.1038/s41467-017-01566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rao E, Hou Y, Huang X, Wang L, Wang J, Zheng W, Yang H, Yu X, Yang K, Bugno J, et al. All-trans retinoic acid overcomes solid tumor radioresistance by inducing inflammatory macrophages. Sci Immunol. 2021;6(60). doi: 10.1126/sciimmunol.aba8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pouget JP, Navarro-Teulon I, Bardiès M, Chouin N, Cartron G, Pèlegrin A, Azria D. Clinical radioimmunotherapy—the role of radiobiology. Nat Rev Clin Oncol. 2011;8(12):720–734. doi: 10.1038/nrclinonc.2011.160. [DOI] [PubMed] [Google Scholar]
- 79.Patel RB, Hernandez R, Carlson P, Grudzinski J, Bates AM, Jagodinsky JC, Erbe A, Marsh IR, Arthur I, Aluicio-Sarduy E, et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci Transl Med. 2021;13(602). doi: 10.1126/scitranslmed.abb3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morris ZS, Guy EI, Werner LR, Carlson PM, Heinze CM, Kler JS, Busche SM, Jaquish AA, Sriramaneni RN, Carmichael LL, et al. Tumor-Specific Inhibition of in situ Vaccination by Distant Untreated Tumor Sites. Cancer Immunol Res. 2018;6(7):825–834. doi: 10.1158/2326-6066.CIR-17-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kerr CP, Grudzinski JJ, Nguyen TP, Hernandez R, Weichert JP, Morris ZS. Developments in combining targeted radionuclide therapies and immunotherapies for cancer treatment. Pharmaceutics. 2022;15(1):128. doi: 10.3390/pharmaceutics15010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magee K, Marsh IR, Turek MM, Grudzinski J, Aluicio-Sarduy E, Engle JW, Kurzman ID, Zuleger CL, Oseid EA, Jaskowiak C, et al. Safety and feasibility of an in situ vaccination and immunomodulatory targeted radionuclide combination immuno-radiotherapy approach in a comparative (companion dog) setting. PLos One. 2021;16(8):e0255798. doi: 10.1371/journal.pone.0255798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kacem H, Almeida A, Cherbuin N, Vozenin MC. Understanding the FLASH effect to unravel the potential of ultra-high dose rate irradiation. Int J Radiat Biol. 2022;98(3):506–516. doi: 10.1080/09553002.2021.2004328. [DOI] [PubMed] [Google Scholar]
- 84.Vozenin MC, Bourhis J, Durante M. Towards clinical translation of FLASH radiotherapy. Nat Rev Clin Oncol. 2022;19(12):791–803. doi: 10.1038/s41571-022-00697-z. [DOI] [PubMed] [Google Scholar]
- 85.Renaudin X. Reactive oxygen species and DNA damage response in cancer. Int Rev Cell Mol Biol. 2021;364:139–161. [DOI] [PubMed] [Google Scholar]
- 86.Iturri L, et al. Proton FLASH radiation therapy and immune infiltration: evaluation in an orthotopic glioma rat model. Int J Radiat Oncol Biol Phys. 2022. [DOI] [PubMed] [Google Scholar]
- 87.Bertho A, Iturri L, Brisebard E, Juchaux M, Gilbert C, Ortiz R, Sebrie C, Jourdain L, Lamirault C, Ramasamy G, et al. Evaluation of the role of the immune system response after minibeam radiation therapy. Int J Radiat Oncol Biol Phys. 2023;115(2):426–439. doi: 10.1016/j.ijrobp.2022.08.011. [DOI] [PubMed] [Google Scholar]
- 88.Diffenderfer ES, Verginadis II, Kim MM, Shoniyozov K, Velalopoulou A, Goia D, Putt M, Hagan S, Avery S, Teo K, et al. Design, implementation, and in vivo validation of a novel proton flash radiation therapy system. Int J Radiat Oncol Biol Phys. 2020;106(2):440–448. doi: 10.1016/j.ijrobp.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morris Z, Dohopolski M, Rahimi A, Timmerman R. Future directions in the use of sabr for the treatment of oligometastatic cancers. Semin Radiat Oncol. 2021;31(3):253–262. doi: 10.1016/j.semradonc.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 90.Moore C, Hsu C-C, Chen W-M, Chen BPC, Han C, Story M, Aguilera T, Pop LM, Hannan R, Fu Y-X, et al. Personalized ultrafractionated stereotactic adaptive radiotherapy (pulsar) in preclinical models enhances single-agent immune checkpoint blockade. Int J Radiat Oncol Biol Phys. 2021;110(5):1306–1316. doi: 10.1016/j.ijrobp.2021.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lhuillier C, Vanpouille-Box C, Galluzzi L, Formenti SC, Demaria S. Emerging biomarkers for the combination of radiotherapy and immune checkpoint blockers. Semin Cancer Biol. 2018;52:125–134. doi: 10.1016/j.semcancer.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang EP, O’Connor JPB, McShane LM, Giger ML, Lambin P, Kinahan PE, Siegel EL, Shankar LK. Criteria for the translation of radiomics into clinically useful tests. Nat Rev Clin Oncol. 2023;20(2):69–82. doi: 10.1038/s41571-022-00707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun R, Limkin EJ, Vakalopoulou M, Dercle L, Champiat S, Han SR, Verlingue L, Brandao D, Lancia A, Ammari S, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19(9):1180–1191. doi: 10.1016/S1470-2045(18)30413-3. [DOI] [PubMed] [Google Scholar]
- 94.Sun R, Sundahl N, Hecht M, Putz F, Lancia A, Rouyar A, Milic M, Carré A, Battistella E, Alvarez Andres E, et al. Radiomics to predict outcomes and abscopal response of patients with cancer treated with immunotherapy combined with radiotherapy using a validated signature of CD8 cells. J ImmunoTher Cancer. 2020;8(2):e001429. doi: 10.1136/jitc-2020-001429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choe K, Hontani Y, Wang T, Hebert E, Ouzounov DG, Lai K, Singh A, Béguelin W, Melnick AM, Xu C. Intravital three-photon microscopy allows visualization over the entire depth of mouse lymph nodes. Nat Immunol. 2022;23(2):330–340. doi: 10.1038/s41590-021-01101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 97.Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre A, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–1203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]


