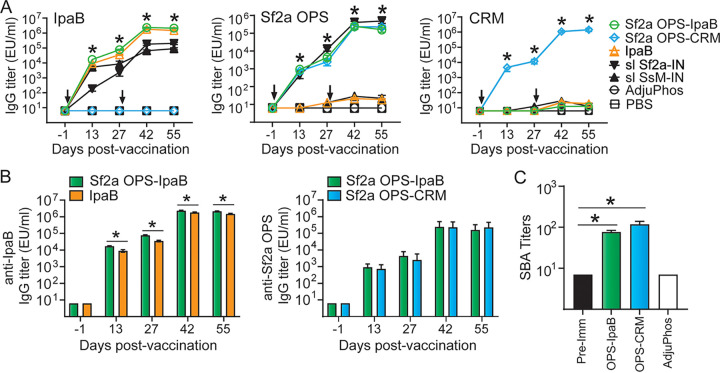
FIG 4.
Immunogenicity of S. flexneri 2a OPS-IpaB conjugate vaccine. Mice (20/group/challenge strain) were immunized intramuscularly (i.m.) on days 0 and 28 with 10 μg of S. flexneri 2a OPS-IpaB, OPS-CRM, or IpaB, each admixed with AdjuPhos. Negative-control groups received PBS or AdjuPhos. Mice immunized intranasally (i.n.) with sublethal doses (105 CFU) of S. flexneri 2a (Sf2a) or S. sonnei (SsM) were included as positive controls for S. flexneri 2a and S. sonnei challenge, respectively. (A) IpaB-, S. flexneri 2a OPS-, and CRM-specific serum IgG measured by ELISA; arrows indicate immunization. Mean of individual titers ± standard error of the mean; *, P < 0.001 versus AdjuPhos and PBS by t test. (B) IpaB- and OPS-IgG titer comparison for specific groups; *, P < 0.01 by t test. (C) S. flexneri 2a serum bactericidal activity (SBA) on day 55. Mean of individual titers ± standard error of the mean; *, P < 0.001 versus prevaccination by t test. sl Sf2a-IN, sublethal dose of S. flexneri 2a-IN; sl SsM-IN, sublethal dose of S. sonnei Moseley-IN.