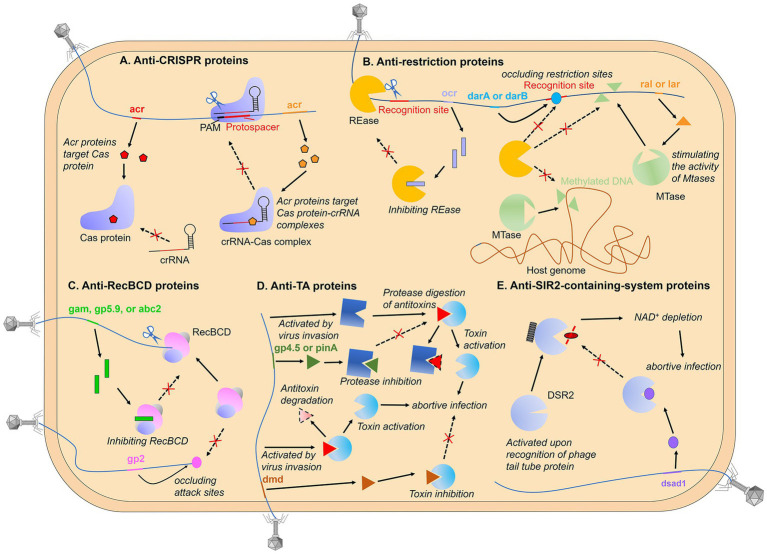
Figure 1.
Phage-encoded proteins that direct bind immune proteins. (A) the host CRISPR-Cas system targets and cleaves the exogenously invading nucleic acid under the guidance of crRNA upon recognizing the PAM of foreign nucleic acid. Phage-encoded Acr proteins directly target Cas proteins or Cas protein-crRNA complexes, inhibiting recognition or cleavage of phage nucleic acids; (B) the restriction-modification (R-M) system is a mechanism that modifies specific sequence motifs in the host genome and degrades unmodified foreign DNA. Phage proteins can circumvent the R-M system through various strategies, such as inhibiting restriction endonucleases (REases), shielding unmodified restriction sites, and stimulating host protein modifications to the phage genome; (C) RecBCD degrades DNA lacking Chi and containing free DNA ends, and phage-encoded proteins can directly bind to RecBCD, preventing cleavage of the phage genome, or they can bind to the ends of linear phage genomes to protect the injected DNA from RecBCD attack; (D) the antitoxin functions to neutralize the toxin during normal bacterial growth and activate toxins during phage propagation. Phage-encoded proteins can mimic antitoxins or prevent the degradation of antitoxins, thereby avoiding the release of toxins; (E) bacterial defense systems with SIR2 domains deplete NAD+ upon phage infection, thus triggering abortive infection. Phage-encoded proteins can bind to SIR2-domain proteins and inactivate their enzymatic activity.