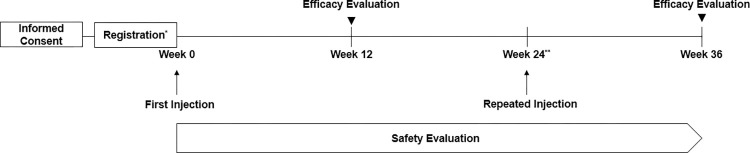
Fig 1. Flow of the study.
* If the criteria for inclusion/exclusion of subjects are met and all necessary information has been collected before the administration, the registration date (the date written consent and permission to use data are obtained) and the administration date may be the same. ** Second injection was at least 6 months after the first injection.