Figure 3: UBXN2A binds to Rictor protein and promotes its proteasomal degradation.
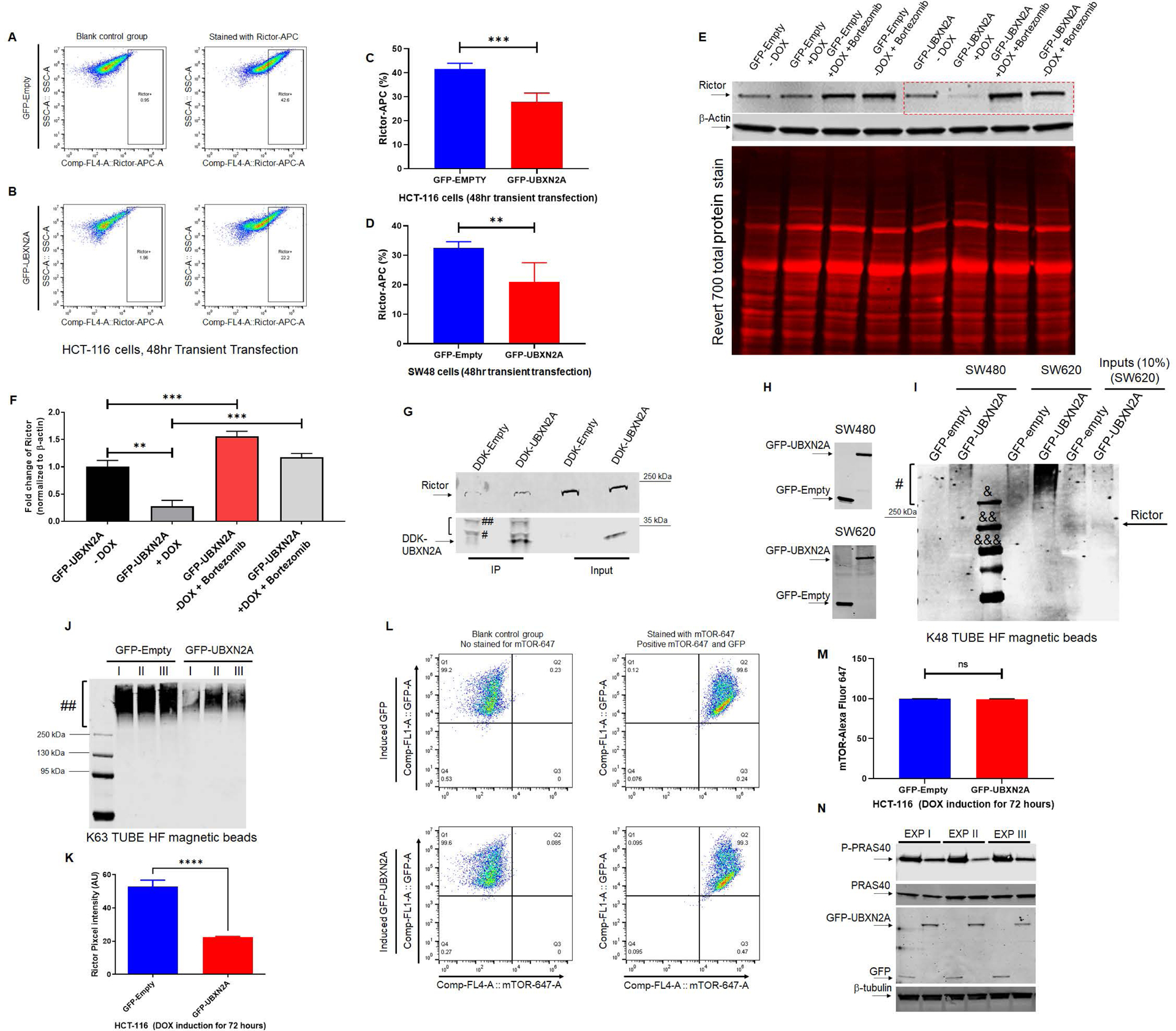
Flowcytometry analysis of HCT-116 cells transiently transfected with GFP-empty or GFP-UBXN2A vectors for 48 hours revealed UBXN2A overexpression significantly decreases Rictor protein (A-C). Similar to the HCT-116 cell line, transient transfection of SW48 cells with GFP-empty or GFP-UBXN2A vectors for 48 hours followed by flow-cytometry analysis showed UBXN2A can significantly decrease the protein level of Rictor (D). Induction of UBXN2A in HCT-116 treated with DOX for 72 hours decreased the half-life of Rictor, while the presence of a proteasome inhibitor (bortezomib, 50nM) rescues rapid turnover of Rictor (red box in panel E; quantitated signals in panel F). A β-actin antibody and Revert 700 total protein stain were used as the loading control. HCT-116 cells were transfected with DDK-Empty or DDK-UBXN2A plasmid for 48 hours followed by immunoprecipitation using anti-DDK antibody immobilized on magnetic IgG beads. DDK-UBXN2A pulled down Rictor protein, indicating UBXN2A binds to Rictor protein. Two bands tagged with one # and two ## are light and heavy chains, respectively (G). Due to the high molecular weight of Rictor protein (220 kDa), we observed a low non-specific affinity of Rictor protein to magnetic beads in control. In another set of experiments, K48 TUBE HF magnetic beads were used to pull down ubiquitinated Rictor in SW480 and SW620 (a metastatic colon cancer cell line) transiently transfected with GFP-empty or GFP-UBXN2A vectors for 48 hours (H). GFP-UBXN2A increases the K48-linked ubiquitination ladder (#) of Rictor protein (I), particularly in SW620. The K48 pull-down experiment was repeated with the same results trend. &, &&, and &&& are protein molecular markers for 250 kDa, 130, kDa, and 95 kDa, respectively. In contrast, K63 magnetic beads revealed induced UBXN2A decreases pulled down K63 linked chain of Rictor protein (n=3, Panels J) due to the reduction of total Rictor protein in the presence of UBXN2A measured by immunofluorescent microscopy in HCT-116 cells (n=150 cells-Panel K). A set of flow-cytometry analyses in HCT-116 cells transiently overexpressing GFP-empty of GFP-UBXN2A proteins showed the presence of UBXN2A has no significant effect on mTOR protein, another protein member of the mTORC2 complex (L and M). Finally, the Tet-on inducible GFP-empty and GFP-UBXN2A were treated with DOX for 72 hours. Cell lysates were subjected to WB using anti-PRAS40 (total protein and P-PRAS40, a phosphorylated form of protein) as well as a GFP antibody. A triplicate WB revealed that UBXN2A decreases the protein level P-PRAS40, activated by the Rictor-mTORC2 signaling pathway (N). Together, these flow-cytometry, K48/K63 linked chains, and WB results indicate UBXN2A selectively targets and degrades Rictor proteins via ubiquitin-proteasome pathway in colon cancer cells (** p< 0.01, *** p< 0.001, mean +/− SD).
