Fig. 4: Absence of UBXN2A leads to elevation of Rictor, resulting in inhibition of mTORC2’s downstream protein targets.
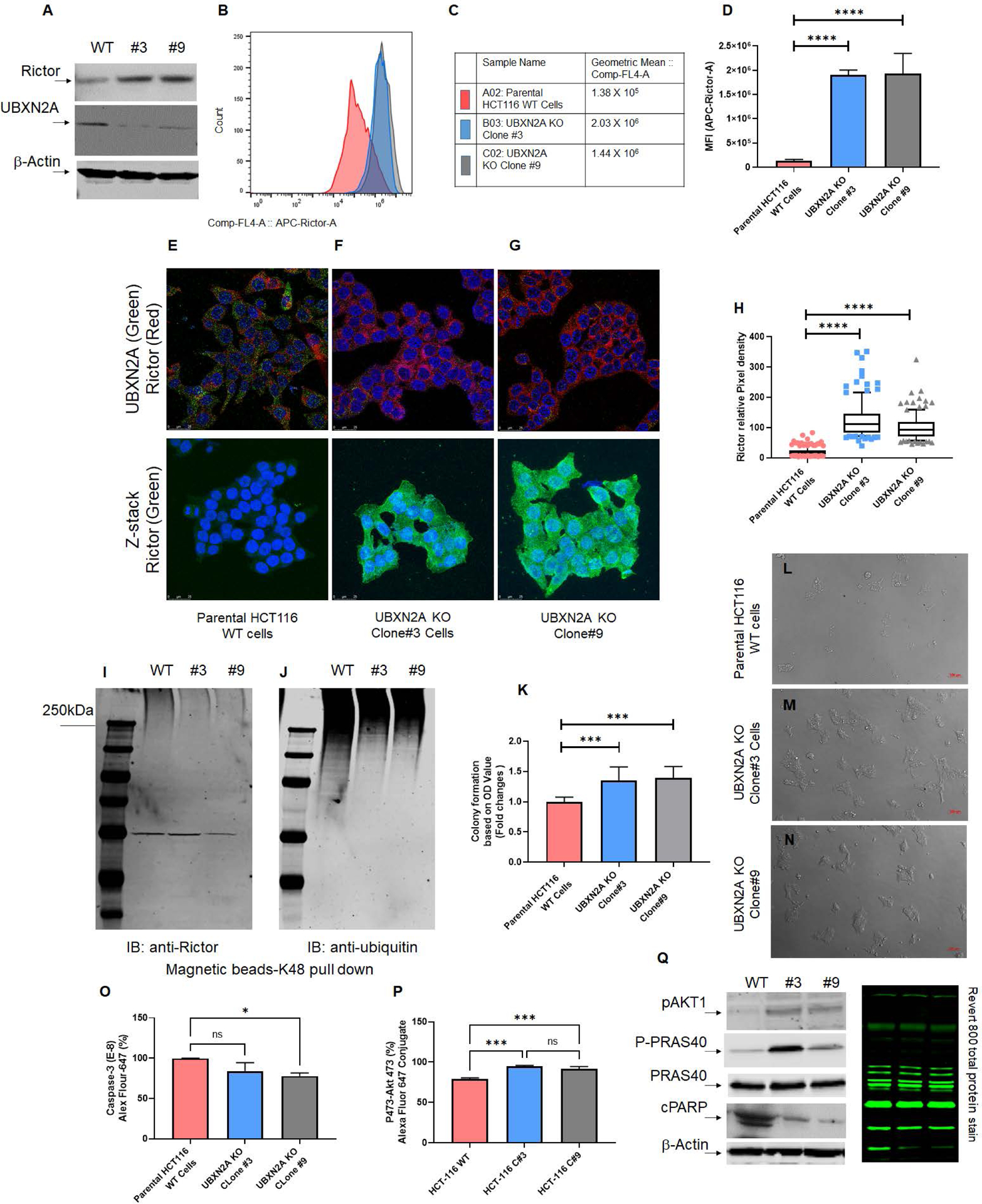
Two stable CRISPR UBXN2A KO HCT-116 cells generated by CRISPR/Cas9 genome editing were validated by WB (A) and flow-cytometry analysis (B-D). Clone 3 and clone 9 UBXN2A KO were subjected to confocal microscopy study (E-H), TUBE K48-linked ubiquitin chain magnetic beads pull-down (I-J), and crystal violet cell viability assay (K-N). These data demonstrate that the absence of UBXN2A in HCT-116 (clones 3 and 9) significantly elevates the level of Rictor protein measured by flow- cytometry and immunocytochemistry (D and H). Furthermore, the absence of UBXN2A decreases the K48 ubiquitinated form of Rictor in HCT-116 cells (I-J). The absence of UBXN2A and simultaneous elevation of Rictor leads to higher cell proliferation and less apoptosis stained and quantitated by crystal violet assay (K-N). Flow-cytometry analysis of Caspase-3 (O) and the elevation of pAKT473 (P) further confirmed the inverse relationship between UBXN2A and Rictor protein levels and Rictor’s downstream pathways responses. A set of WB experiments revealed that the absence of UBXN2A (clones 3 and 9) leads to the elevation of pAKT1 and P-PRAS40, as well as the reduction of cleaved PARP (cPARP), which are regulated by the activated mTORC2 pathway (Q). A β-actin antibody and Revert 800 total protein stain were used as the loading control (* n≥3, p<0.05, ***p<0.001, ****p<0.0001, mean ± SD).
