Figure. 5: UBXN2A expression leads to the reduction of VEGF proteins in metastatic colon cancer cells.
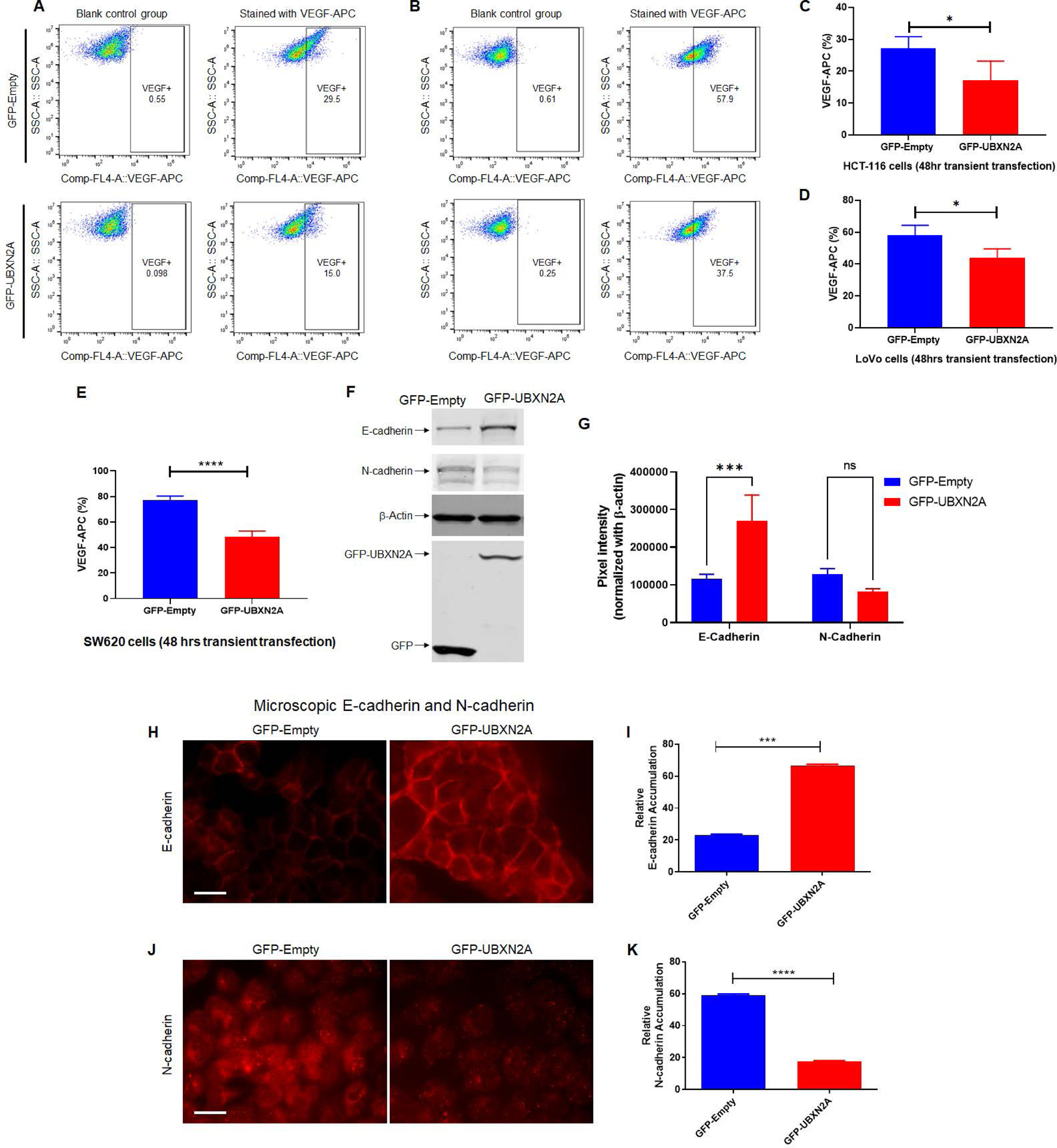
To determine the effects of UBXN2A expression on the VEGF protein level, we transiently transfected HCT-116 (A and C) and LoVo (B and D) colon cancer cells with GFP-empty and GFP-UBXN2A. Flow-cytometry experiments revealed that GFP-UBXN2A and not GFP-Empty decreases the level of VEGF proteins (C and D). A similar significant reduction of VEGF was measured in SW620 metastatic colon cancer cells (n=4, E). Cell lysates of HCT-116 cells transiently transfected with GFP-empty or GFP-UBXN2A were subjected to WB and probed with anti-E-cadherin and anti-N-cadherin antibodies (F). The quantitation of E-cadherin and N-cadherin bands normalized by β-actin in WB results (n=3, G). To confirm that UBXN2A regulates functional E-cadherin and N-Cadherin at the plasma membrane, we conducted a set of immunocytochemistry experiments (H and J). Measured fluorescent signals (n=150 cells per group, I and K) revealed UBXN2A overexpression can switch the E-cadherin and N-cadherin which potentially suppress EMT. These results indicate that UBXN2A expression can directly interfere with the two major metastatic pathways (angiogenesis and EMT) activated by the Rictor-mRORC2 pathway (* p<0.05, ***p<0.001, ****p<0.0001, mean ± SD).
