Figure. 6: Genetic and pharmacological regulation of UBXN2A suppress colon cancer migration.
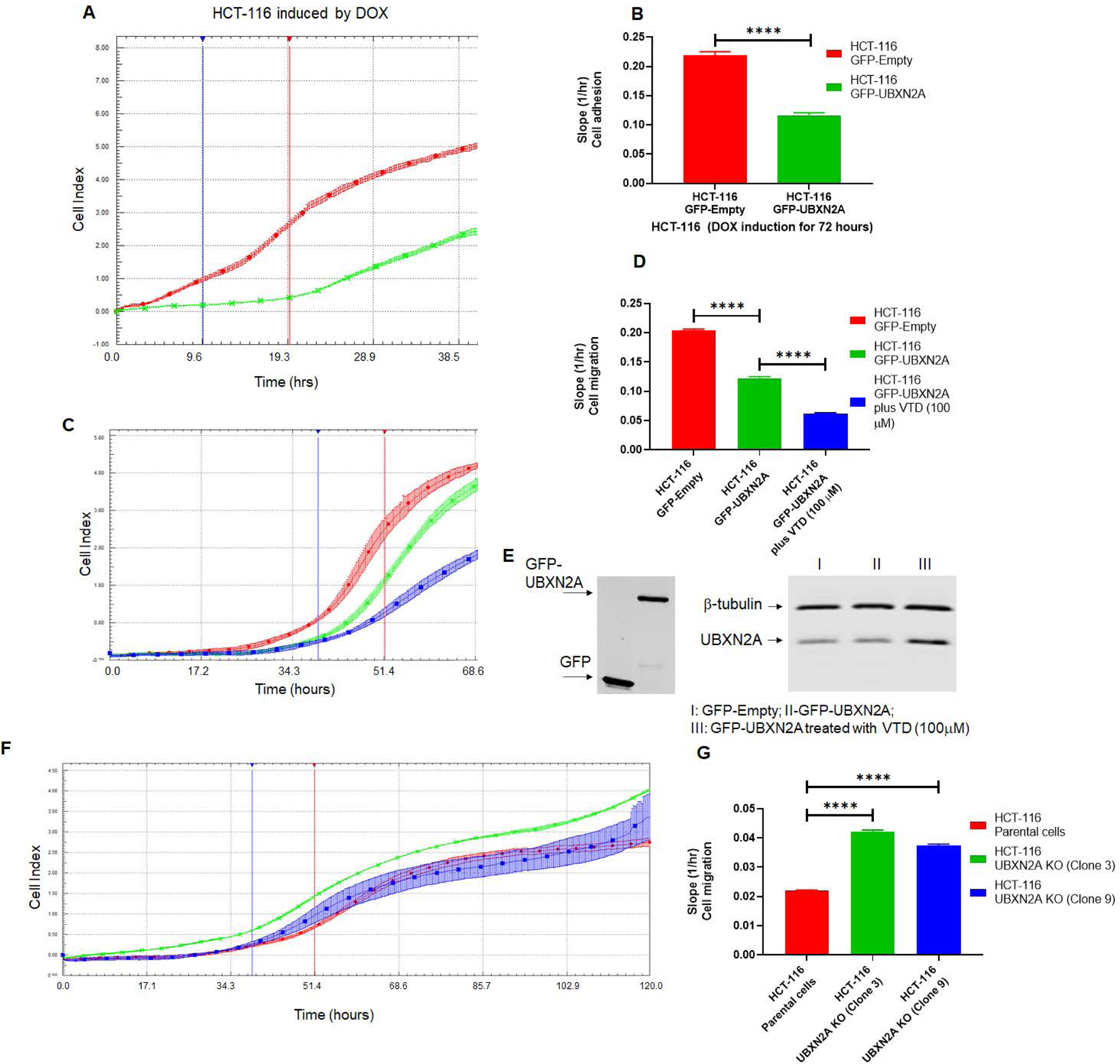
HCT-116 GFP-empty or GFP-UBXN2A treated with DOX for 72 hours, were plated in 16-wells E-plate or CIM-plate (xCELLigence Real-Time technology) and monitored in real-time for cell adhesion (A-B) and migration (C-D). A and C are representative graphs comparing the rate of adhesion and migration using the calculated cell index (see methods section). B and D show calculated slopes for these two events during critical time points marked with blue and red lines in the A and C diagrams. Interestingly, enhancement of UBXN2A endogenous protein by the pharmacological tool, veratridine, further decreases HCT-116 cancer cell migration (D, blue column versus green column). E shows equal overexpression of exogenous GFP and GFP-UBXN2A in HCT-116 with the Tet-on promoter system and elevated endogenous UBXN2A in veratridine-treated cells subjected to xCELLigence analysis. To examine the physiological effect of UBXN2A in cell migration, HCT-116 UBXN2A KO cells (clones 3 and 9) were subjected to a set of xCELLigence migration assays (F). Results shown in G indicate that the UBXN2A’s loss of function leads to significant cell migration indicating that the negative interaction of UBXN2A with Rictor affects downstream signaling pathways regulated by the mTORC2-Rictor pathway. Experiments were repeated two times with N of 4 per cell line per experiment (****P<0.0001, mean ± SD).
