Background:
As the SARS-CoV-2-induced pandemic wanes, a substantial number of patients with acute Corona Virus-induced disease (COVID-19 continue to have symptoms for a prolonged time after initial infection. These patients are said to have postacute sequelae of COVID (PASC) or “long COVID”. The underlying pathophysiology of this syndrome is poorly understood and likely quite heterogeneous. The role of persistent, possibly deviant inflammation as a major factor in comorbidity is suspected.
Objective:
To review data that address the relative importance of inflammation in the pathophysiology spectrum of PASC and to address how this would impact diagnosis and approach to therapy in patients identified as having such inflammatory abnormalities.
Methods:
A review of public databases, including PubMed, MeSH, NLM catalog, and clinical trial databases such as clinicaltrials.gov.
Results:
The literature supports a prominent role for various forms and types of inflammation in the pathophysiologic spectrum of PASC. Such inflammation can be persistent ant CoV-2-specific responses, new onset autoimmune responses, or a loss of normal immunoregulation resulting in widespread, sustained inflammatory pathologies that can affect both broad constitutional symptoms (such as fatigue, neurocognitive dysfunction, and anxiety/depression) and organ-specific dysfunction and/or failure.
Conclusions:
PASC is a significant clinical entity with similarities to and differences from other postviral syndromes. Significant research efforts are ongoing to better understand specific aberrant inflammatory pathways present in individual patients for the purpose of developing and implementing effective therapies and ultimately prophylaxis strategies to prevent the progression of COVID-19 as well as likely future viral illnesses and pandemics.
Keywords: COVID-19, inflammation, pathophysiology, postacute sequelae of COVID
1. Introduction
The devastation in lives, national and international economies, and societies caused by coronavirus disease 2019 (COVID-19) over the past 3+ years has caused varying levels of social disarray and destruction on a global scale [1]. The morbidity and mortality risk for the illness caused worldwide shutdowns that have had a severe adverse clinical impact. According to the World Health Organization, as of mid-May, 2023, there have been over 765 million reported global COVID cases, with almost 7 million deaths. Even though the pandemic has been declared ended as of May 11, 2023, there are still almost one-half million new cases per week still being reported [2].
Fortunately, the overwhelming majority of COVID patients recover and return to their previous state of health within several weeks after infection. A small percentage die of acute illness. As the SARS-CoV-2 (CoV-2) virus-induced acute phase moves from pandemic to endemic status, there are still lasting clinical effects for a significant minority of infected individuals who have never recovered completely (Fig. 1). This condition, termed postacute sequelae of COVID-19 (PASC), also known as “long COVID,” has afflicted a large number of patients, variably estimated at between 10% and 40% of the acutely infected populations [3]. Given the highly limited understanding of the pathophysiology and the growing consensus that clinical manifestations vary significantly both between and within distinct patient populations, the enigmas of effectively caring for these patients confront health care providers regularly. This review is focused on the role that aberrant inflammation may play in at least some patients with PASC and provides a theoretical rationale for diagnosis and treatment of the inflammatory mechanisms that may cause or at least contribute to PASC pathophysiology.
Figure 1.
Clinical outcomes from developing COVID-19. The major outcomes throughout the pandemic have consistently been that the majority of patients who develop COVID-19 resolve their acute symptoms with little or no long-term comorbidities. The mortality rate from acute infection has dropped significantly during the course of the pandemic but still occurs in susceptible patients. The rate of postacute sequelae of COVID-19 (PASC) may be diminishing but still affects an alarming percentage of patients who developed acute COVID-19.
2. Challenges from PASC for researchers and clinicians
The definition of PASC has evolved over the past 3 years but was actually initially reported shortly after the beginning of the pandemic [4]. These initial patients were largely characterized by their constitutional components—persistent, often debilitating fatigue [5], neurocognitive dysfunction including concentration and attention deficits as well as what is commonly called “brain fog” [6], and signs and symptoms from end-organ dysfunction [7] (Table 1). These signs and symptoms lasted far longer than the 7–14 days typically associated with the acute illness. Initially, the American definition was based upon clinical signs and symptoms that last for 30 days or more after initial infection whereas the World Health Organization definition utilized a 120-day after-initial presentation window [8]. As time has progressed, clinicians and researchers are working diligently towards establishing a consensus definition [9]. Current efforts are centering around the duration of new or persistent symptoms for 2 months that occur 120 days or longer after the last infection and cannot be otherwise explained by an alternate diagnosis [10]. The PASC definition is also evolving since a significant number of patients have recurrent episodes of new COVID symptoms that may or may not completely resolve over the subsequent several weeks.
The reported clinical phenotypes of PASC vary widely [11]. Estiri et al. [12] used a nonhospitalized PASC cohort and identified 33 distinct phenotypes. This further complicates the definition as not all PASC patients have all the clinical manifestations. What does seem to be common among patients with PASC are the components of fatigue, neurocognitive dysfunction, anxiety, depression, muscle pain and/or weakness, and dyspnea [13]. Other symptoms, that are either exacerbated (if preexistent before initial CoV-2 infection) or new onset, include major organ dysfunction such as lung inflammation and fibrosis [14], cardiac dysfunction such as acute myocardial infarction and myocarditis [15], hepatitis [16], kidney failure [17], new-onset diabetes mellitus [18] and other less common organ-specific manifestations [19]. Multiple reports indicate the common presence of dysautonomias which can manifest as postural orthostatic hypotension, cardiac dysrhythmias, gastrointestinal motility disorders, and others [20]. Other clinical manifestations suggest a mast cell activation syndrome (MCAS)-type picture in some patients [21].
Although proposed from mostly retrospective studies, certain risk factors for developing PASC have been observed [22]. These include gender (female), age (over 50), severe acute COVID-19, obesity, lifestyle factors such as smoking, immune compromised status (rheumatologic, organ transplant recipients, and oncology patients, etc.), type 2 diabetes, and multiple other chronic medical conditions [23]. Significant research efforts have been invested into correlating clinical risk factors with specific phenotypes [24]. No specific consensus has yet been reached to provide associations that might be useful clinically to rapidly diagnose and treat specific patients. Efforts are currently ongoing to generate regional and ultimately global consensus on definitions for the purpose of understanding pathophysiologies and developing effective therapies.
3. Inflammation and PASC
Multiple theories have been advanced to explain the pathophysiology of PASC. Emerging data indicate that PASC is actually a syndrome rather than a specific disease entity due to the multiple clinical phenotypes, variable duration of symptoms, and response to specific interventions [25]. The majority of PASC patients appear to recover partial or full baseline function over time (weeks to a few months). However, significant numbers of patients continue to have debilitating symptoms for months and now years after initial presentation [26, 27]. The origin of these dysfunctions may vary from direct CoV-2-mediated viral damage (with consequent organ parenchymal destruction and/or dysfunction) [28] to reactivation of latent viruses (such as Epstein Barr virus) which can produce their own symptom spectrum due to defined pathophysiology [29] to hemostasis/coagulation pathway abnormalities causing thrombotic events even remotely from the initial presentation [30]. Multiple reports indicate that an alarming number of patients have evidence of persistent CoV-2 viremia long after initial presentation. This appears to be more common in PASC patients than those COVID patients who have totally recovered from their acute illness.
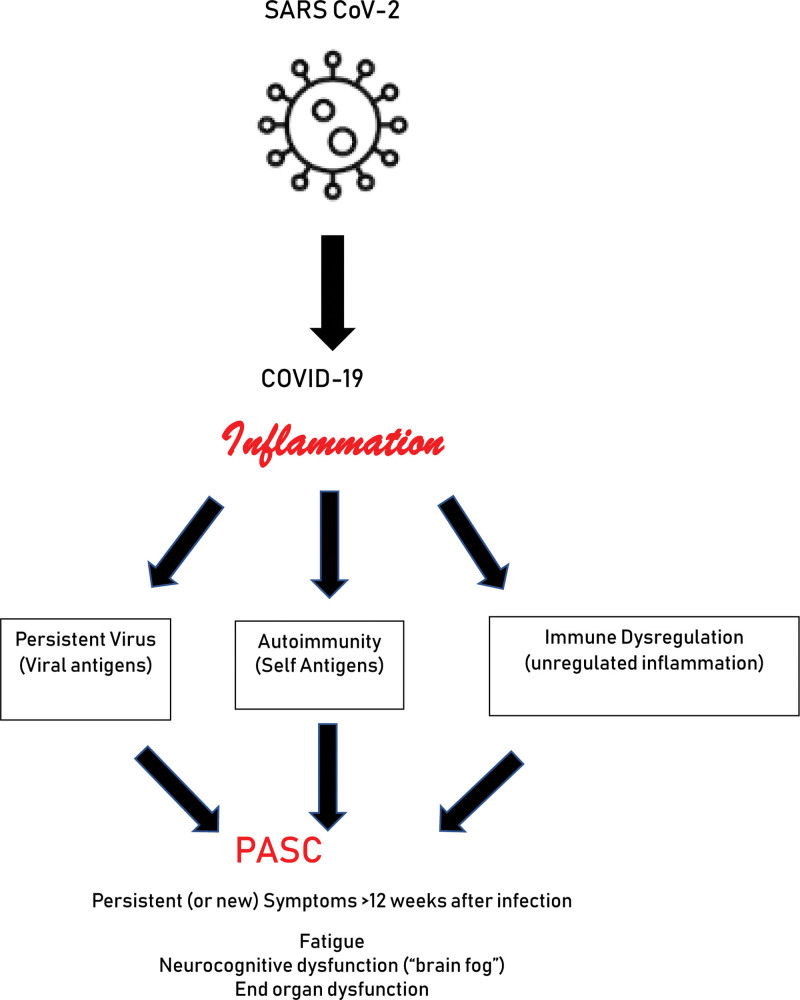
What is common with all (or at least most) proposed pathophysiological mechanisms of PASC is the effects of aberrant inflammation [31]. This may well include excessive inflammation during the initial infection which was proposed as a major morbidity and mortality risk factor in acute COVID, especially earlier in the pandemic [32], that causes end-organ damage, particularly cardiopulmonary. As data emerge in the phenotyping of PASC patients, the continued presence of signs, symptoms, and biomarkers typically associated with inflammatory disorders past the acute phase of COVID is commonly observed [33]. Accordingly, three different mechanisms related to inflammation have emerged to explain at least some of the signs and symptoms of PASC (Fig. 2). These include continued innate and adaptive immune responses to persistent CoV-2 virion and/or viral peptides [34], autoimmune mechanisms from loss of normal regulatory networks and/or molecular mimicry [35], and a more generalized loss of immunoregulatory pathways which allows persistent and/or excessive adaptive and innate inflammation with subsequent pathological consequences [36]. It is likely that these mechanisms represent more of a spectrum rather than distinct categories.
Figure 2.
Persistent inflammation hypothesis for PASC. In this model, patients who develop COVID-19 and continue to have symptoms associated with the acute illness (either worsening or preexisting comorbidities or new onset) for at least 12 weeks with no other clinical explanation. The persistent inflammation may be a result of a prolonged innate and/or adaptive immune response to viral antigen from sequestered SARS-CoV-2, autoimmunity against a variety of self-antigens that may or may not have rheumatologic presentations, and/or a generalized dysregulation of multiple immune mechanisms resulting in unregulated, persistent inflammatory changes. Clinical manifestations may be constitutional such as persistent, debilitating fatigue and/or neurocognitive dysfunction including concentration and attention deficits as well as “brain fog or a variety of inflammatory mediated end organ dysfunctions.
3.1. Immune response against persistent CoV-2 antigens
The presence of persistent CoV-2 viral antigens has been reported since shortly after the beginning of the pandemic [37]. Initially, these positive tests were largely considered to be due to infectious virions, and prolonged isolation was proposed until PCR tests became negative. As the pandemic progressed, studies emerged that showed evidence of persistent virion and/or viral antigen in various body fluids and tissues from both asymptomatic and PASC patients [38, 39], typically at a frequency higher than that seen in fully recovered COVID patients. The presence of viremia in PASC patients, particularly those who show persistent clinical biomarkers of excessive inflammation and adaptive immunity, could be a target for intervention with specific antiviral compounds [40] or more general interventions that increase host antiviral resilience [41].
3.2. Autoimmune mechanisms
The similarities between clinical manifestations of PASC and those of many distinct autoimmune diseases are remarkable [42]. Patients who develop PASC have been reported to develop new-onset autoimmune disorders, including systemic lupus erythematosus, immune thrombocytopenia purpura, autoimmune thyroid diseases (Grave’s and Hashimoto’s), Guillain-Barre syndrome, and others [43]. The mechanisms responsible for this association in these patients have not been defined but several possibilities have been advanced. A fundamental mechanism that has long been proposed is molecular mimicry [44]. When viral proteins share some homogeneity with self-proteins, aberrant self-reactive clones can emerge through cross-reactive sensitization or become active through dysregulation [45]. Candidate molecules have included some heat shock proteins (HSP) [46] which, when the individual cells are stressed by the effects of CoV-2 infection, can initiate autoimmune sensitization [47]. Marino Gammazza et al. [48] compared CoV-2 proteins with human HSP seeking the degree of homogeneity. They did sequence analysis on over 20,000 human proteins and compared them to the protein libraries of CoV-2 virions. There were 3781 human proteins that shared at least six amino acid sequences with CoV-2 proteins. Of those, 17 were molecular chaperones consistent with HSP. This would be a plausible explanation for various autoimmune manifestations in many patients with PASC. Other mechanisms involved with autoimmune diseases relate to complement activation by immune complexes that may occur with viral as well as autoantigen-induced antibodies binding in critical ratios (relative antigen access) to induce inflammatory organ pathology as well as many of the observed constitutional symptoms characteristic of PASC.
3.3. Generalized immunoregulatory dysfunction
There are still PASC patients who do not fit into the persistent CoV-2 infection or autoimmune categories because of a lack of diagnostic criteria yet still show signs, symptoms, and biomarkers of persistent inflammation [13]. Other viral illnesses have been associated with nonspecific immune activation of both innate and adaptive mechanisms [49]. Accordingly, in acute illness, inflammatory phenomena such as cytokine storms can create significant morbidity and mortality both acutely [50] and with longer-term sequelae [51]. Production of inflammatory biomarkers such as interleukin (IL)-1β, IL-6 and tumor necrosis factor-α, IL-10, and transforming growth factor-β can all contribute to both innate and adaptive immune dysregulation with subsequent persistent inflammatory damage.
3.4. Mast cells and PASC
Mast cells (MCs) are innate immune cells located in various tissues throughout the body, particularly plentiful in and about mucosal surfaces. They possess multiple cell-surface receptors which react to various stimuli and, after activation and subsequent degranulation, release multiple mediators either immediately from preformed granules within a few hours or a longer interval associated with new mediator synthesis. These mediators include histamine, heparin, various cytokines, prostaglandins, leukotrienes, and proteases, particularly, various isoforms of tryptase [52]. There is a spectrum of clinical symptoms associated with MC activity, from simple allergic rhinitis to various forms of asthma, allergic skin diseases, and systemic reactions to generalized MC degranulation called anaphylaxis. Clinical symptoms associated with excess numbers of MC, either system-wide or in specific organs, are termed mastocytosis [53]. However, there is a category of illnesses associated with varying clinical symptoms of MC activity termed MCAS. In the various clinical presentations of MCAS, excessive amounts of MC mediators are released in response to a variety of triggers that may be clinically evident or hidden (idiopathic). Biomarker profiles have been proposed to differentiate various forms of MCAS [54]. Such classifications are useful for optimal diagnosis, accurate prognosis, and optimal therapies. Some PASC patients have presented with clinical manifestations of excess MC activity, including pruritus and urticarial-type rashes [55], GI symptoms including nausea, vomiting, and abdominal cramping with or without diarrhea [56], and others. It has been hypothesized that, in susceptible patients, COVID may have exacerbated existing (but undiagnosed) MCAS [57]. It has also been suggested that COVID can produce sustained activation of previously normal MC owing to the persistence of viral particles with subsequent but ongoing MC activation due to complement activation from CoV-2-antibody immune complexes [58]. It is tantalizing to consider the possibilities associated with therapies directed at excessive MC activity in patients whose clinical presentation supports the approach. Various combinations of antihistamines (either H1 or H2 antagonists or inverse agonists), leukotriene modifiers, MC stabilizers, and/or systemic corticosteroids may all play therapeutic roles in some of these PASC patients [59, 60].
3.5. Potential role of CoV-2-specific vaccines in PASC
No discussion about aberrant inflammatory pathways in a postviral syndrome like PASC would be complete without a mention of the possible role of vaccines. Varying reports (usually very small studies or case series) have provided varying perspectives about the risk for PASC after CoV-2-specific vaccines, with some reporting increased risk and others reporting protection [61]. Ceban et al. [62] recently reported a systematic review and meta-analysis of the relative risk for vaccinated vs unvaccinated COVID patients to develop PASC. Their analysis demonstrated that, for over 250,000 patients assessed, the odds ratio for vaccinated patients progressing to PASC was 0.539, indicating a significant protective effect. Further, for individuals who already had PASC and were subsequently vaccinated, no significant adverse effects of the vaccine were evident. These data may be useful going forward to encourage vaccination (and likely booster vaccines) to actually prevent PASC, particularly in at-risk populations. Further, it would suggest that those who already have (or had) PASC should be reassured of the lack of increased risk of subsequent vaccination.
4. Approach to identifying pathophysiology in specific patient groups
Given the clinical sign and symptom similarities that occur in PASC patients, identifying specific pathophysiology in specific groups of patients as well as individuals is a daunting challenge. It is important to develop the necessary approaches to ultimately provide guidelines and evidence-based support to clinicians who care for these patients. However, many of the specific biomarker assessments for inflammation described above are not readily available in clinical settings. There are several options that can provide some direction as to the underlying inflammatory pathophysiology contributing to the morbidity of PASC in individual patients.
Perhaps the issue that can be addressed most readily is assessing the level of inflammation in individual PASC patients. There are multiple clinical tests that have abnormal results associated with a state of persistent inflammation [63]. These include assessments for C-reactive protein, erythrocyte sedimentation rate, D-dimer, procalcitonin, D-dimer, IL-6, troponin, and creatine kinase. Persistent elevations in these levels can suggest the presence of generalized or organ-specific damage with subsequent inflammation.
Assessment for autoimmune biomarkers can be accomplished in a focused fashion based on clinical presentation. Autoantibody blood panels, beginning with an assessment for the presence of antinuclear antibodies and subsequent specific autoantibodies such as anti-dsDNA, anti-Sm, and antiphospholipid antibodies often grouped by clinical laboratories as a lupus panel. Disease activity can be assessed by clinical symptoms and assessment of complement components (C3, C4) as these are often decreased with increased disease activity. For patients with significant constitutional symptoms, screens for thyroid function along with antithyroid antibodies (antithyroid peroxidase, antithyroglobulin, and antithyroid stimulating hormone receptor) may be useful.
Susceptibility and clinical course of PASC in immune-compromised patients have been reported to be more severe, and prognostic outcomes are more guarded [64]. Thus, it is reasonable to assess the overall immune competence of each individual PASC patient, including total isotype levels (IgG, IgA, and IgM), complement screen (CH50 or CH100), complete blood count to confirm adequate numbers of immune cells, determination of total T cell (CD3), as well as subpopulations (CD4, CD8), NK (CD56), and B cells (CD20). Abnormalities should be further investigated in the context of new types of infections since initially developing COVID, serological evidence of recurrent herpesvirus (HSV, CMV, EBV, and VZV). If an immune deficiency is discovered, then it becomes imperative to address the mechanism and determine whether the immunodeficiency occurred before or after the initial CoV-2 infection.
5. General therapeutic principles to address suspected inflammation in PASC
The challenge of a therapeutic plan for PASC is that most care has been largely supportive with the hope and expectation that the patient will eventually improve over weeks to months [23]. Of course, if there is significant end-organ dysfunction that can be identified, focused therapy to address the organ pathology is appropriate. However, when PASC patients present with new onset or exacerbated conditions such as fatigue, malaise, neurocognitive dysfunction, and dysautonomia, often little if any definitive therapy can be offered which is itself a source of anxiety and frustration for patients and families [65].
In terms of addressing the inflammatory components of PASC, if evidence of persistent CoV-2 antigens is present, an effort for aggressive antiviral therapy may be useful. Studies (directed by Sing et al at Stanford University in California USA) are underway to determine whether treatment with nirmatrelvir + ritonavir (Paxlovid) for 15 days (as opposed to the usual 5 days for an acute infection) can have positive effects on symptoms in PASC patients [66]. A similar study, led by Krumholz et al., is ongoing at Yale University in New Haven, CT, USA looking at the effects of 15 days of nirmatrelvir + ritonavir compared to ritonavir placebo on scores from the PROMIS-29 questionnaire reflecting overall clinical condition [67]. Clinical trials for PASC using other antivirals that target either the CoV-2 virus or reactivated herpesviruses associated with PASC have been the subject of multiple reports. Results are mixed possibly due to variations between study populations, stages (duration) of PASC pathophysiology, and distinct therapeutic agents being tested. Significant global research efforts are attempting to define the role of specific antivirals in the treatment of PASC. Similar approaches for the treatment of autoimmunity in PASC patients are aimed at lowering the accompanying inflammatory profiles typically observed in other patients with autoimmune disorders not associated with COVID or PASC. While this does not specifically address the role of CoV-2 directly, it is an approach designed to induce or hasten the achievement of clinical control in these patients. Given that we are only slightly over 3 years since the pandemic began, it will take time to determine the nature of new-onset autoimmune diseases post-PASC compared to standard cases that are part of our clinical care systems and how these new cases behave over prolonged periods of time.
Studies have also been initiated to address more generalized inflammation not specifically associated with a single etiology in PASC patients. A clinical trial (PLEXCOVIL), led by Bennani et al. from France, is studying the effects of plasmapheresis on constitutional symptoms of PASC patients, including fatigue, postexertional malaise, dyspnea, headache, myalgia, neurocognitive impairment, anosmia/ageusia, and anxiety/depression as 3 months and 6 months after study initiation [68]. A clinical trial led by our group collaborating with nine other US medical centers is examining the role of Immulina [69], a commercially available dietary supplement derived from the Spirulina cyanobacterium with reported immunomodulatory properties, on inflammatory biomarker profiles (C-reactive protein, D-dimer, and IL-6) in PASC patients experiencing persistent fatigue, neurocognitive impairment, and/or dyspnea. The 8-week placebo-controlled study seeks to determine whether Immulina can normalize the inflammatory biomarker profile and whether such changes correlate with clinical symptom questionnaires [68].
Given the recognition of inflammation that plays a significant (if not always primary) role in PASC pathophysiology, it is not unexpected that studies have been performed to assess the potential clinical value of immunosuppressive agents. An ongoing phase 2 trial is investigating the effects of RSLV-132, an IV-administered enzymatically active ribonuclease designed to digest the RNA in autoantibodies and immune complexes to render them inert and (presumably) nonpathogenic in PASC patients, on fatigue as measured by the PROMIS Fatigue 7a questionnaire after 71 days [70]. Another currently active trial from Cleveland Hospital is examining the anti-inflammatory effects of vitamins K2 (MK-7) and D3 in PASC patients who show blood evidence of increased inflammatory mediators. Primary outcomes are changes in serum inflammatory biomarkers correlated with blood levels of vitamins K2 and D3 as well as clinical PASC symptom assessment [71].
6. Future directions
Both clinicians and researchers agree that substantial percentages of patients who have developed acute COVID-19 go on to develop PASC of varying severity with variable degrees of morbidity for varying periods of time. The majority of PASC patients appear to resolve their symptoms over several months but a substantial proportion still have symptoms months and now years later. There is no consensus on an approach to care for these patients other than a supportive approach with the hope that their symptoms will ultimately resolve. Given the relatively short duration of the pandemic, it is not yet clear whether there will be an increased mortality risk for PASC patients but questions such as this need to be addressed. Building consensus definitions for the spectrum of this illness is still in its infancy, and, accordingly, an approach to diagnosis and therapy is still under development. Large registry studies such as the US NIH-funded RECOVER (REsearching COVid to Enhance Recovery) study are designed to gather large amounts of prospective and retrospective longitudinal information on PASC patients compared to fully recovered COVID patients and COVID-negative controls. The study is aimed at understanding risk factors for PASC compared to non-PASC patients and prospectively following acutely infected COVID patients to determine the factor(s) that may lead to progression to d/or the factors that would protect against developing PASC. The study design will ultimately be based upon principles of precision medicine that will look for molecular-omics-based patterns that are impacted by environmental influences and personal choices that could impact risk.
In the interval, until such studies produce actionable data, the approach to diagnosis and management should seek to identify underlying pathologies as potential therapeutic targets. This is particularly true for inflammation-based pathophysiologies. Learning lessons from other postviral syndromes [72] can inform therapeutic directions for the ultimate benefit of afflicted patients.
Acknowledgements
This work is supported in part by grants U54GM115428, OT2HL161847, and NCT 05524532 from the National Institutes of Health.
Conflict of interest
The authors have no financial conflicts of interest.
Footnotes
Published online 13 June 2023
References
- 1.Miyah Y, Benjelloun M, Lairini S, Lahrichi A. COVID-19 impact on public health, environment, human psychology, global socioeconomy, and education. Sci World J 2022;2022:5578284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus (COVID-19) Dashboard. http://covid19.who.int last accessed May 12, 2023.
- 3.Hope AA, Evering TH. Postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. Infect Dis Clin North Am 2022;36:379-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanichkachorn G, Newcomb R, Cowl CT, Murad MH, Breeher L, Miller S, Trenary M, Neveau D, Higgins S. Post-COVID-19 Syndrome (Long Haul Syndrome): description of a multidisciplinary clinic at Mayo clinic and characteristics of the initial patient cohort. Mayo Clin Proc 2021;96:1782-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Bharti S, Garg I. Post COVID fatigue: can we really ignore it? Indian J Tuberc 2022;69:238-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, Rodrigues NB, Subramaniapillai M, Di Vincenzo JD, Cao B, Lin K, Mansur RB, Ho RC, Rosenblat JD, Miskowiak KW, Vinberg M, Maletic V, McIntyre RS. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: a systematic review and meta-analysis. Brain Behav Immun 2022;101:93-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chippa V, Aleem A, Anjum F. Post Acute Coronavirus (COVID-19) syndrome. 2023. Feb 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [PubMed] [Google Scholar]
- 8.O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, Karamchandani U, Simms-Williams N, Cassambai S, Ardavani A, Wilkinson TJ, Hawthorne G, Curtis F, Kingsnorth AP, Almaqhawi A, Ward T, Ayoubkhani D, Banerjee A, Calvert M, Shafran R, Stephenson T, Sterne J, Ward H, Evans RA, Zaccardi F, Wright S, Khunti K. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. E Clin Med 2022;55:101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022;22:e102-e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai CC, Hsu CK, Yen MY, Lee PI, Ko WC, Hsueh PR. Long COVID: an inevitable sequela of SARS-CoV-2 infection. J Microbiol Immunol Infect 2023;56:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenny G, Townsend L, Savinelli S, Mallon PWG. Long COVID: clinical characteristics, proposed pathogenesis and potential therapeutic targets. Front Mol Biosci 2023;10:1157651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estiri H, Strasser ZH, Brat GA, Semenov YR, Patel CJ, Murphy SN; Consortium for Characterization of COVID-19 by EHR (4CE). Evolving phenotypes of non-hospitalized patients that indicate long COVID. BMC Med 2021;19:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherif ZA, Gomez CR, Connors TJ, Henrich TJ, Reeves WB; RECOVER Mechanistic Pathway Task Force. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife 2023;12:e86002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barash M, Ramalingam V. Post-COVID interstitial lung disease and other lung sequelae. Clin Chest Med 2023;44:263-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sewanan LR, Clerkin KJ, Tucker NR, Tsai EJ. How does COVID-19 affect the heart? Curr Cardiol Rep 2023;25:171-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi RB, Wu ZH. Association between COVID-19 and chronic liver disease: mechanism, diagnosis, damage, and treatment. World J Virol 2023;12:22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Copur S, Berkkan M, Basile C, Tuttle K, Kanbay M. Post-acute COVID-19 syndrome and kidney diseases: what do we know? J Nephrol 2022;35:795-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wrona M, Skrypnik D. New-onset diabetes mellitus, hypertension, dyslipidaemia as sequelae of COVID-19 infection-systematic review. Int J Environ Res Public Health 2022;19:13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenny G, McCann K, O’Brien C, Savinelli S, Tinago W, Yousif O, Lambert JS, O'Broin C, Feeney ER, De Barra E, Doran P, Mallon PWG; All-Ireland Infectious Diseases (AIID) Cohort Study Group. Identification of distinct long COVID Clinical phenotypes through cluster analysis of self-reported symptoms. Open Forum Infect Dis 2022;9:ofac060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dani M, Dirksen A, Taraborrelli P, Torocastro M, Panagopoulos D, Sutton R, Lim PB. Autonomic dysfunction in “long COVID”: rationale, physiology and management strategies. Clin Med (Lond) 2021;21:e63-e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arun S, Storan A, Myers B. Mast cell activation syndrome and the link with long COVID. Br J Hosp Med (Lond) 2022;83:1-10. [DOI] [PubMed] [Google Scholar]
- 22.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ 2021;374:n1648. [DOI] [PubMed] [Google Scholar]
- 23.Astin R, Banerjee A, Baker MR, Dani M, Ford E, Hull JH, Lim PB, McNarry M, Morten K, O'Sullivan O, Pretorius E, Raman B, Soteropoulos DS, Taquet M, Hall CN. Long COVID: mechanisms, risk factors and recovery. Exp Physiol 2023;108:12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, Gokhale KM, Taverner T, Chandan JS, Brown K, Simms-Williams N, Shah AD, Singh M, Kidy F, Okoth K, Hotham R, Bashir N, Cockburn N, Lee SI, Turner GM, Gkoutos GV, Aiyegbusi OL, McMullan C, Denniston AK, Sapey E, Lord JM, Wraith DC, Leggett E, Iles C, Marshall T, Price MJ, Marwaha S, Davies EH, Jackson LJ, Matthews KL, Camaradou J, Calvert M, Haroon S. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med 2022;28:1706-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ledford H. Long-COVID treatments: why the world is still waiting. Nature 2022;608:258-260. [DOI] [PubMed] [Google Scholar]
- 26.Files JK, Sarkar S, Fram TR, Boppana S, Sterrett S, Qin K, Bansal A, Long DM, Sabbaj S, Kobie JJ, Goepfert PA, Erdmann N. Duration of post-COVID-19 symptoms is associated with sustained SARS-CoV-2-specific immune responses. JCI Insight 2021;6:e151544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danesh V, Arroliga AC, Bourgeois JA, Boehm LM, McNeal MJ, Widmer AJ, McNeal TM, Kesler SR. Symptom clusters seen in adult COVID-19 recovery clinic care seekers. J Gen Intern Med 2023;38:442-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol 2020;51:613-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vojdani A, Vojdani E, Saidara E, Maes M. Persistent SARS-CoV-2 infection, EBV, HHV-6 and other factors may contribute to inflammation and autoimmunity in long COVID. Viruses 2023;15:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Toro A, Bozzani A, Tavazzi G, Urtis M, Giuliani L, Pizzoccheri R, Aliberti F, Fergnani V, Arbustini E. Long COVID: long-term effects? Eur Heart J Suppl 2021;23(Suppl E):E1-E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.PHOSP-COVID Collaborative Group. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med 2022;10:761-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubio-Rivas M, Mora-Luján JM, Formiga F, Arévalo-Cañas C, Lebrón Ramos JM, Villalba García MV, Fonseca Aizpuru EMª, Díez-Manglano J, Arnalich Fernández F, Romero Cabrera JL, García García GM, Pesqueira Fontan PM, Vargas Núñez JA, Freire Castro SJ, Loureiro Amigo J, de Los Reyes Pascual Pérez M, Alcalá Pedrajas JN, Encinas-Sánchez D, Mella Pérez C, Ena J, Gracia Gutiérrez A, Esteban Giner MJ, Varona JF, Millán Núñez-Cortés J, Casas-Rojo J-M; SEMI-COVID-19 Network. WHO ordinal scale and inflammation risk categories in COVID-19. Comparative study of the severity scales. J Gen Intern Med 2022;37:1980-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maamar M, Artime A, Pariente E, Fierro P, Ruiz Y, Gutiérrez S, Tobalina M, Díaz-Salazar S, Ramos C, Olmos JM, Hernández JL. Post-COVID-19 syndrome, low-grade inflammation and inflammatory markers: a cross-sectional study. Curr Med Res Opin 2022;38:901-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giménez-Orenga K, Pierquin J, Brunel J, Charvet B, Martín-Martínez E, Perron H, Oltra E. HERV-W ENV antigenemia and correlation of increased anti-SARS-CoV-2 immunoglobulin levels with post-COVID-19 symptoms. Front Immunol 2022;13:1020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Churilov LP, Normatov MG, Utekhin VJ. Molecular mimicry between SARS-CoV-2 and human endocrinocytes: a prerequisite of post-COVID-19 endocrine autoimmunity? Pathophysiology 2022;29:486-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García-González P, Tempio F, Fuentes C, Merino C, Vargas L, Simon V, Ramirez-Pereira M, Rojas V, Tobar E, Landskron G, Araya JP, Navarrete M, Bastias C, Tordecilla R, Varas MA, Maturana P, Marcoleta AE, Allende ML, Naves R, Hermoso MA, Salazar-Onfray F, Lopez M, Bono MR, Osorio F. Dysregulated immune responses in COVID-19 patients correlating with disease severity and invasive oxygen requirements. Front Immunol 2021;12:769059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang HP, Sun YL, Wang YF, Yazici D, Azkur D, Ogulur I, Azkur AK, Yang Z-W, Chen X-X, Zhang A-Z, Hu J-Q, Liu G-H, Akdis M, Akdis CA, Gao Y-D. Recent developments in the immunopathology of COVID-19. Allergy 2023;78:369-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cherne MD, Gentry AB, Nemudraia A, Nemudryi A, Hedges JF, Walk H, Blackwell K, Snyder DT, Jerome M, Madden W, Hashimi M, Sebrell TA, King DB, Plowright RK, Jutila MA, Wiedenheft B, Bimczok D. Severe acute respiratory syndrome coronavirus 2 is detected in the gastrointestinal tract of asymptomatic endoscopy patients but is unlikely to pose a significant risk to healthcare personnel. Gastro Hep Adv 2022;1:844-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanson BA, Visvabharathy L, Ali ST, Kang AK, Patel TR, Clark JR, Lim PH, Orban ZS, Hwang SS, Mattoon D, Batra A, Liotta EM, Koralnik IJ. Plasma biomarkers of neuropathogenesis in hospitalized patients with COVID-19 and those with postacute sequelae of SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm 2022;9:e1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peluso MJ, Anglin K, Durstenfeld MS, Martin JN, Kelly JD, Hsue PY, Henrich TJ, Deeks SG. Effect of oral nirmatrelvir on long COVID symptoms: 4 cases and rationale for systematic studies. Pathog Immun 2022;7:95-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aricò E, Bracci L, Castiello L, Urbani F, Casanova JL, Belardelli F. Exploiting natural antiviral immunity for the control of pandemics: lessons from Covid-19. Cytokine Growth Factor Rev 2022;63:23-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang KT, Hsu BC, Chen DY. Autoimmune and rheumatic manifestations associated with COVID-19 in adults: an updated systematic review. Front Immunol 2021;12:645013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boaventura P, Macedo S, Ribeiro F, Jaconiano S, Soares P. Post-COVID-19 condition: where are we now? Life (Basel) 2022;12:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vahabi M, Ghazanfari T, Sepehrnia S. Molecular mimicry, hyperactive immune system, and SARS-COV-2 are three prerequisites of the autoimmune disease triangle following COVID-19 infection. Int Immunopharmacol 2022;112:109183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol 2020;217:108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mantej J, Bednarek M, Sitko K, Świętoń M, Tukaj S. Autoantibodies to heat shock protein 60, 70, and 90 are not altered in the anti-SARS-CoV-2 IgG-seropositive humans without or with mild symptoms. Cell Stress Chaperones 2021;26:735-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucchese G, Flöel A. SARS-CoV-2 and Guillain-Barré syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress Chaperones 2020;25:731-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marino Gammazza A, Légaré S, Lo Bosco G, Fucarino A, Angileri F, Conway de Macario E, Macario AJ, Cappello F. Human molecular chaperones share with SARS-CoV-2 antigenic epitopes potentially capable of eliciting autoimmunity against endothelial cells: possible role of molecular mimicry in COVID-19. Cell Stress Chaperones 2020;25:737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zubchenko S, Kril I, Nadizhko O, Matsyura O, Chopyak V. Herpesvirus infections and post-COVID-19 manifestations: a pilot observational study. Rheumatol Int 2022;42:1523-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol 2021;93:250-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silva Andrade B, Siqueira S, de Assis Soares WR, de Souza Rangel F, Santos NO, Dos Santos Freitas A, Ribeiro da Silveira P, Tiwari S, Alzahrani KJ, Góes-Neto A, Azevedo V, Ghosh P, Barh D. Long-COVID and Post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses 2021;13:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galli SJ, Gaudenzio N, Tsai M. Mast cells in inflammation and disease: recent progress and ongoing concerns. Annu Rev Immunol 2020;38:49-77. [DOI] [PubMed] [Google Scholar]
- 53.Tzankov A, Duncavage E, Craig FE, Kelemen K, King RL, Orazi A, Quintanilla-Martinez L, Reichard KK, Rimsza LM, Wang SA, Horny H-P, George TI. Mastocytosis. Am J Clin Pathol 2021;155:239-266. [DOI] [PubMed] [Google Scholar]
- 54.Jackson CW, Pratt CM, Rupprecht CP, Pattanaik D, Krishnaswamy G. Mastocytosis and mast cell activation disorders: clearing the air. Int J Mol Sci 2021;22:11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kocatürk E, Salman A, Cherrez-Ojeda I, Criado PR, Peter J, Comert-Ozer E, Abuzakouk M, Agondi RC, Al-Ahmad M, Altrichter S, Arnaout R, Arruda LK, Asero R, Bauer A, Ben-Shoshan M, Bernstein JA, Bizjak M, Boccon-Gibod I, Bonnekoh H, Bouillet L, Brzoza Z, Busse P, Campos RA, Carne E, Conlon N, Criado RF, de Souza Lima EM, Demir S, Dissemond J, Doğan Günaydin S, Dorofeeva I, Ensina LF, Ertaş R, Ferrucci SM, Figueras-Nart I, Fomina D, Franken SM, Fukunaga A, Giménez-Arnau AM, Godse K, Gonçalo M, Gotua M, Grattan C, Guillet C, Inomata N, Jakob T, Karakaya G, Kasperska-Zając A, Katelaris CH, Košnik M, Krasowska D, Kulthanan K, Kumaran MS, Lang C, Larco-Sousa JI, Lazaridou E, Leslie TA, Lippert U, Llosa OC, Makris M, Marsland A, Medina IV, Meshkova R, Palitot EB, Parisi CAS, Pickert J, Ramon GD, Rodríguez-Gonzalez M, Rosario N, Rudenko M, Rutkowski K, Sánchez J, Schliemann S, Sekerel BE, Serpa FS, Serra-Baldrich E, Song Z, Soria A, Staevska M, Staubach P, Tagka A, Takahagi S, Thomsen SF, Treudler R, Vadasz Z, Valle SOR, Van Doorn MBA, Vestergaard C, Wagner N, Wang D, Wang L, Wedi B, Xepapadaki P, Yücel E, Zalewska-Janowska A, Zhao Z, Zuberbier T, Maurer M. The global impact of the COVID-19 pandemic on the management and course of chronic urticaria. Allergy 2021;76:816-830. [DOI] [PubMed] [Google Scholar]
- 56.Weinstock LB, Brook JB, Walters AS, Goris A, Afrin LB, Molderings GJ. Mast cell activation symptoms are prevalent in Long-COVID. Int J Infect Dis 2021;112:217-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wechsler JB, Butuci M, Wong A, Kamboj AP, Youngblood BA. Mast cell activation is associated with post-acute COVID-19 syndrome. Allergy 2022;77:1288-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee MH, Perl DP, Steiner J, Pasternack N, Li W, Maric D, Safavi F, Horkayne-Szakaly I, Jones R, Stram MN, Moncur JT, Hefti M, Folkerth RD, Nath A. Neurovascular injury with complement activation and inflammation in COVID-19. Brain 2022;145:2555-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malone RW, Tisdall P, Fremont-Smith P, Liu Y, Huang X-P, White KM, Miorin L, Moreno E, Alon A, Delaforge E, Hennecker CD, Wang G, Pottel J, Blair RV, Roy CJ, Smith N, Hall JM, Tomera KM, Shapiro G, Mittermaier A, Kruse AC, García-Sastre A, Roth BL, Glasspool-Malone J, Ricke DO. COVID-19: famotidine, histamine, mast cells, and mechanisms. Front Pharmacol 2021;12:633680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glynne P, Tahmasebi N, Gant V, Gupta R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med 2022;70:61-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023;21:133-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ceban F, Kulzhabayeva D, Rodrigues NB, Di Vincenzo JD, Gill H, Subramaniapillai M, Lui LMW, Cao B, Mansur RB, Ho RC, Burke MJ, Rhee TG, Rosenblat JD, McIntyre RS. COVID-19 vaccination for the prevention and treatment of long COVID: a systematic review and meta-analysis. Brain Behav Immun 2023;111:211-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.García-Abellán J, Fernández M, Padilla S, García JA, Agulló V, Lozano V, Ena N, García-Sánchez L, Gutiérrez F, Masiá M. Immunologic phenotype of patients with long-COVID syndrome of 1-year duration. Front Immunol 2022;13:920627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dioverti V, Salto-Alejandre S, Haidar G. Immunocompromised patients with protracted COVID-19: a review of “Long Persisters”. Curr Transplant Rep 2022;9:209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 2021;53:737-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Study # NCT05576662 http://clinicaltrials.gov last accessed May 12, 2023.
- 67.Study # NCT05668091 http://cliicaltrials.gov last accessed May 12, 2023
- 68. Study # NCT05543590 http://clinicaltrials.gov last accessed May 12, 2023.
- 69.Pugh ND, Edwall D, Lindmark L, Kousoulas KG, Iyer AV, Haron MH, Pasco DS. Oral administration of a Spirulina extract enriched for Braun-type lipoproteins protects mice against influenza A (H1N1) virus infection. Phytomedicine 2015;22:271-276. [DOI] [PubMed] [Google Scholar]
- 70. Study # NCT04944121 http://clinicaltrials.gov last accessed May 12, 2023.
- 71. Study # NCT05356936 http://clinicaltrials.gov last accessed May 12, 2023.
- 72.Wong TL, Weitzer DJ. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)-a systemic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas) 2021;57:418. [DOI] [PMC free article] [PubMed] [Google Scholar]