Abstract
Background and Hypothesis
Negative symptom trajectory in clinical high risk (CHR) for psychosis is ill defined. This study aimed to better characterize longitudinal patterns of change in negative symptoms, moderators of change, and differences in trajectories according to clinical subgroups. We hypothesized that negative symptom course will be nonlinear in CHR. Clinical subgroups known to be more severe variants of psychotic illness—deficit syndrome (DS), persistent negative syndrome (PNS), and acute psychosis onset—were expected to show more severe baseline symptoms, slower rates of change, and less stable rates of symptom resolution.
Study Design
Linear, curvilinear, and stepwise growth curve models, with and without moderators, were fitted to negative symptom ratings from the NAPLS-3 CHR dataset (N = 699) and within clinical subgroups.
Study Results
Negative symptoms followed a downward curvilinear trend, with marked improvement 0–6 months that subsequently stabilized (6–24 months), particularly among those with lower IQ and functioning. Clinical subgroups had higher baseline ratings, but distinct symptom courses; DS vs non-DS: more rapid initial improvement, similar stability of improvements; PNS vs non-PNS: similar rates of initial improvement and stability; transition vs no transition: slower rate of initial improvement, with greater stability of this rate.
Conclusions
Continuous, frequent monitoring of negative symptoms in CHR is justified by 2 important study implications: (1) The initial 6 months of CHR program enrollment may be a key window for improving negative symptoms as less improvement is likely afterwards, (2) Early identification of clinical subgroups may inform distinct negative symptom trajectories and treatment needs.
Keywords: symptom course, growth curve analysis, cognition, functioning
Introduction
Negative symptoms are a critical treatment target among those at clinical high risk (CHR) for psychosis for several reasons. First, they are highly prevalent, with at least 1 moderate-severe symptom occurring in 82% of CHR participants.1,2 Second, they are an early clinical marker of psychosis risk that sometimes emerges before attenuated positive symptoms.3 Third, negative symptoms are a significant predictor of both functional decline and transition to a full psychotic disorder.4–11 Unfortunately, attempts to remediate negative symptoms in CHR have been met with limited success.12 To improve early identification and intervention of negative symptoms in CHR, it will be critical to determine when (ie, which timepoint in the high-risk period), where (ie, period of illness where symptoms are declining, plateauing, or entering a critical window), and how (ie, which potential moderators to target at certain timepoints) to focus these efforts.13 As it stands, this endeavor is complicated by inconsistent past findings on the trajectory of negative symptoms in CHR and the heterogeneous nature of the negative syndrome itself.
Prior studies examining the trajectory of negative symptoms in CHR have produced inconsistent results. For example, subgroups with higher vs lower negative symptoms have been detected at varying base rates (eg, 9% vs 42.5%).14,15 Subsequently, the subgroups differ in how much and how rapidly their negative symptoms change. For example, a subgroup of CHR individuals with baseline moderate-severe negative symptoms continued to show elevated symptoms at 6- and 12-month assessments.15 Conversely, other studies observed a linear trajectory where negative symptoms improved both rapidly and modestly across time.14–17 Moderators of the base rates and degree of negative symptom change have also been predicted by different variables across CHR studies. Most studies found that more severe base rates and persistently elevated negative symptoms were associated with worse functional outcomes.8,15 Poorer cognition, increased defeatist beliefs, persistent depressive symptoms, older age, and full transition to psychosis have generally been associated with worse and persistent negative symptoms, though reports are mixed.8,14,15,18 A lack of consensus in past findings may result in part from the study design and analytic approaches used. Past studies inferred symptom trajectories based on small samples, different symptom monitoring timeframes, and infrequent assessment points, precluding examination of possible complex, nonlinear longitudinal patterns, and moderators of these patterns. Prior methods, such as generalized estimating equations or generalized linear mixed models, do not allow for the operationalization of several clinically informative parameters such as the initial slope of change (velocity), how the initial slope changes over time (acceleration), if there are plateaus in symptom change, and whether it is possible to detect key windows where symptom change specifically occurred. Growth curve modeling can operationalize these constructs and allow for testing of moderators, but this analytic approach has yet to be systematically applied in CHR.
Furthermore, negative symptoms are remarkably heterogeneous in phenomenology and etiology. On negative symptom structured interviews, 2 participants can receive the same score on an item for very different reasons.19 For example, 1 case may receive a “moderate” rating on avolition because they are depressed and lack motivation for goal-directed activities. Others might fail to engage in activities because anxiety or persecutory delusions make them fear leaving the house. However, it is possible to have avolition even in the absence of all these factors for reasons that are unknown but assumed to be due to the neurobiological processes inherent to psychotic disorders. Sometimes negative symptoms resulting from these causes are transient and last only days to weeks, whereas others are enduring trait-like features that last months to years.20 Several tools have been developed to deconstruct the heterogeneity of negative symptoms among adults with schizophrenia that may also hold promise for CHR populations. Foremost among these are algorithmic tools for deriving subgroups referred to as the “deficit syndrome” (DS) and “persistent negative symptoms” (PNS).21–23 These tools can be effective proxies for assessment instruments requiring detailed clinical information that may not always be available and thereby exclude many cases from research evaluation.
The DS is defined as negative symptoms of moderate or greater severity that are primary or idiopathic and enduring (≥12 months).19 Approximately 32% of schizophrenia patients meet criteria for DS,24 which has been proposed as a separate disease within the broader diagnosis of schizophrenia that has distinct symptoms, risk factors, etiology, course, and treatment response.19,25 Other negative symptom cases, not considered primary, are designated as “secondary” and can result from a variety of other clinical factors (eg, depression, hallucinations, antipsychotic sedation).26 The DS proxy algorithm tool has yet to be validated in the CHR population; however, past CHR studies indicate it is a warranted avenue to explore as a liberal definition of at least moderate negative symptoms in the absence of depression has classified 32.7% patients as primary, paralleling the schizophrenia literature.24,27 If the DS can be validly detected among those at CHR, these individuals would be expected to display greater stability of moderate-severe levels of negative symptoms and perhaps greater rates of transition to psychosis since the DS is a more severe variant of the illness in many ways. PNS are similar to the DS in that they are enduring (≥6 months) but differ by being more broadly defined and can result from either primary or secondary factors (instead of only primary factors in DS).20 In schizophrenia, PNS have heterogeneous etiological profiles and are treatment nonresponsive.28 The PNS categorization has been extended to the CHR population, using measures like the SIPS to identify periods where individuals sustain moderate or greater negative symptoms across multiple timepoints.15 CHR cases with PNS evidence more severe and persistent functional impairment than those without. Reports thus far indicate the base rate of PNS in CHR is low (9%) and non-PNS groups do not seem to differ in rate of transition.15 Given the clinical significance of the PNS and DS statuses in CHR, it would be imperative to examine their precise longitudinal trajectories to identify key timepoints of intervention.
As demonstrated by the DS and PNS concepts (summarized in table 1), there are multiple pathways to negative symptoms in CHR. To guide future identification and intervention efforts in CHR, it will be necessary to examine the longitudinal trajectory of these clinically homogeneous subgroups using statistical models capable of capturing meaningful points of change, plateau, and improvement. The current study addressed this need using growth curve modeling applied to the NAPLS-3 dataset to achieve 3 specific aims:
Table 1.
Summary of Clinical Subgroup Definitions
| Persistent Negative Symptoms | Deficit Syndrome | Transition to Psychosis Status |
|---|---|---|
| - Moderate-severe - Can result from primary or secondary factors (ie, depression) - Persistent in short term (≥6 mo) - Treatment non-responsive |
- Moderate-severe - Primary (idiopathic) - Persistent in long term (≥12 mo) - Suspected to be separate, severe variant of psychotic illness |
- At least 1 SOPS Attenuated Psychosis Syndrome item reaching intensity rating of 6 for a frequency of ≥1 h/d for 4 d/wk during the past month or that symptoms seriously impacted functioning |
Note: SOPS, Scale of Psychosis Risk Symptoms.
Aim 1: To identify the model that best fits the longitudinal pattern of negative symptom change in the NAPLS-3 sample and determine moderators of growth curve parameters. Both linear and curvilinear models were evaluated to identify patterns of symptom change, and stepwise models evaluated the presence of windows where negative symptom change specifically occurred. Past studies evaluating CHR samples tended to find that negative symptoms followed a linear trend whereby symptoms seemingly improved at a steady rate over time.14–17 Furthermore, several moderators have been commonly associated with worse and persistent negative symptoms (depression, positive symptoms, cognition, functioning, age8,14,15,18). Thus, with sophisticated growth curve analysis and more frequent assessment points in our study, we hypothesized that curvilinear or stepwise models would provide the best fit for the longitudinal negative symptom data, which would be characterized by a rapid initial improvement in severity followed by a gradual plateauing (Hypothesis 1A). We hypothesized that depression, positive symptoms, cognition, functioning, and age would moderate features of the negative symptom trajectory observed in the CHR sample (Hypothesis 1B). Specifically, we hypothesized that more severe depression, more severe positive symptoms, poorer cognition, poorer functioning, and older age would all predict more severe baseline values; higher levels of cognition and functioning would predict greater longitudinal stability of improved negative symptoms, whereas increased positive symptoms and depression (2 common and transient, secondary sources of negative symptoms) would predict short-term persistence of worse negative symptoms followed by a rapid change in slope across time (ie, more rapid improvement).
Aim 2: To examine whether two negative symptom subgroup statuses (DS, PNS) moderated the longitudinal trajectory of negative symptoms. DS and PNS are hypothesized to differ from those without by showing worse features of negative symptom trajectories, such as having higher baseline scores (intercepts) and more stable, moderate-severe negative symptoms across time (greater velocity and lower acceleration).
Aim 3: To determine whether CHR who transition differ from those that do not transition in their negative symptom trajectory. CHR participants who transition to psychosis are hypothesized to evidence higher baseline scores (intercepts) and greater longitudinal stability of worse negative symptoms across time (greater velocity and lower acceleration).
Methods
Participants
This study reports on data from the North American Prodrome Longitudinal Study (NAPLS-3).29 In NAPLS-3, 806 participants met criteria for a psychosis-risk syndrome on the Structured Interview for Psychosis-Risk Syndromes (SIPS) version 5.6.30 Exclusion criteria included: (1) lifetime DSM-5 Axis I psychotic disorder, (2) IQ <70, (3) a history of a central nervous system disorder, or (4) psychosis-risk symptoms that were better explained by an Axis I disorder. CHR participants (N = 699) who had sufficient negative symptom data were included in analyses.
Materials and Procedures
The NAPLS-3 research protocols were approved by institutional review boards at all participating sites and included informed consent or parent consent when applicable. Trained raters conducted assessments at baseline (initial study ascertainment) and every 2 months for the first 8 months. Additional follow-up assessments occurred at 12, 18, and 24 months. Participants who transitioned to psychosis received an assessment approximately at the point of transition and were followed up 1 year later. The NAPLS-3 clinical measures of interest to the current study were the Scale of Psychosis-Risk Symptoms (SOPS),30 the Presence of Psychotic Symptoms criteria (POPS),31 the Calgary Depression Scale for Schizophrenia (CDSS),32 the Wechsler Abbreviated Scale of Intelligence-2 (WASI-II), and the Global Functioning Social and Role scales (GF:S and GF:R).33,34
Negative Symptoms
The SOPS includes 6 negative symptom items: social anhedonia (N1), avolition (N2), expression of emotion (N3), experience of emotions and self (N4), ideational richness (N5), and occupational functioning (N6). The SOPS N items best fit a 1-factor model (see table S1, Supplementary Data Content 1, for psychometric results on the negative symptom assessment).
Clinical Subgroup Classification
Table 1 summarizes the defining characteristics of clinical subgroups. DS classification was based on a method previously established in schizophrenia,22,35 whereby a score identified individuals with elevated negative symptoms and low affective symptom severity. The sum of the SOPS dysphoria (G2) and stress (G4) symptoms was subtracted from the sum of N1, N2, and N3 symptoms. Resulting scores were z-transformed and percentile cutoff scores were used to psychometrically identify cases with putative primary (above the 75th percentile; n = 171) “DS group” vs secondary negative symptoms (below the 50th percentile; n = 401) “non-DS group.” Twenty-one participants with incomplete baseline symptom assessment were excluded from this calculation. The DS and non-DS groups were then compared on several baseline variables and revealed key clinical differences that supported the validity of the DS categorization in the current CHR sample (see table S2, Supplementary Data Content 2).
The main criteria for PNS in schizophrenia include at least moderate severity of negative symptoms, absence or low levels of depressive symptoms, and demonstrated clinical stability for an extended period (≥6 months).20 Accordingly, PNS was defined in CHR as having 1 N item (N1, N2, or N3) scored as moderately severe to extreme (SOPS rating ≥4) for a duration of 6 months.15 The current study adopted this algorithm to identify PNS (n = 56) and “non-PNS” (n = 478) groups and compared the groups on several baseline variables for validation purposes (see table S3, Supplementary Data Content 3).
The POPS criteria were utilized to determine transition to psychosis. A participant was considered part of the transition group when at least one of the 5 SOPS Attenuated Psychosis items reached a psychotic level of intensity (rating of 6) for a frequency of greater than or equal to 1 hour per day for 4 days per week during the past month or that symptoms seriously impacted functioning. Baseline characteristics of the transition (n = 70) vs the non-transition groups (n = 629) are reported in table S4, Supplementary Data Content 4.
Statistical Analyses
Growth curve models were used to evaluate study hypotheses. Models were fit using the sum of SOPS negative symptom items with baseline assessment coded as time zero. The growth intercept was treated as a latent factor with the loading of negative symptoms at each time fixed to one, while slope (trajectory) was treated as a latent factor with the loading of negative symptoms at each time fixed according to the specific model being tested. For the linear model, slope loadings increased by one at each time from a baseline loading of zero. For the curvilinear model, an additional term was estimated with a quadratic increase of loadings at each time (ie, 0, 1, 4, 9, etc.). The curvilinear model returns 2 parameters of interest: velocity (the initial slope of change) and acceleration (how that initial slope changes over time).
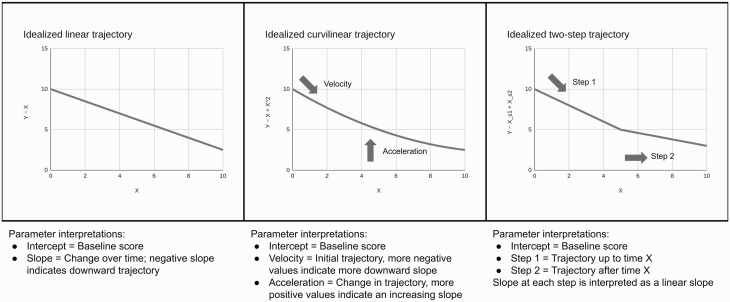
Two stepwise linear models were estimated, a 2-step model with 1 slope from 0 to 6 months and a second slope from 6 to 24 months, while the 3-step model included a third slope from months 12 to 24. For the free-loading model, only baseline and 24-month loadings were fixed (to 0 and 1, respectively) and other loadings were estimated from the data. These models serve to identify different periods of change and may provide superior fit to a single linear slope (which assumes a constant rate of change) or curvilinear, quadratic equation (which assumes a constant rate of change in linear slope, including an ultimate reversal of initial direction). Collectively, the range of models allow for a comprehensive evaluation of possible symptom trajectories including across early, middle, and later timepoints. All growth models included covariances among the intercept and all slopes. See figure 1 for hypothetical visualizations of linear, curvilinear, and stepwise linear trajectory models.
Fig. 1.
Hypothetical linear, curvilinear, and stepwise growth curve models.
Models of differences in trajectory by relevant baseline patient characteristics (depressive symptoms, cognitive functioning, functioning, age), and clinical subgroups (DS, PNS, transition) explored moderation of the best fitting growth curve intercept and slope by the moderator of interest. Moderators were entered as correlated predictors of negative symptom intercept and slope terms within the SEM framework to provide an interpretation similar to coefficients in multiple regression. Full Information Maximum Likelihood was used for all models except where transition was entered as an exogenous variable to account for missing data. Whether transition predicted latent growth trajectory was estimated using the probit link function and pairwise maximum likelihood with missing data handled through pairwise deletion. Fit indices were evaluated for each model, including χ2, comparative fit index (CFI), Tucker-Lewis index (TLI), Akaike information criteria (AIC), Bayesian information criteria (BIC), root-mean-square error of approximation (RMSEA), and standardized root mean squared residual (SRMR). Greater χ2 values indicate worse fit but are heavily influenced by sample size, CFI >0.95 indicates strong fit; TLI >0.95 indicates strong fit; AIC is a relative index of fit; BIC is a relative index of fit; RMSEA <0.08 indicates adequate fit; SRMR <0.08 indicates good fit.36–40 As sensitivity analyses, latent growth curve models were also evaluated as mixed-effects models.41
Results
Table 2 describes the baseline characteristics of the full sample (N = 699) and clinical subgroups. The following subgroups were involved in analyses: DS (n = 171), non-DS (n = 401), PNS (n = 56), non-PNS (n = 478), transition (n = 70), and no transition (n = 629). For categorization agreement of subgroups, see Text, Supplementary Data Content 5.
Table 2.
Full Sample and Clinical Subgroup Characteristics at Baseline
| Full Sample | DS | PNS | Transition | |
|---|---|---|---|---|
| N | 699 | 171 | 56 | 70 |
| Age | 18.19 (4.06) | 17.91 (3.69) | 18.38 (3.51) | 18.74 (3.98) |
| Sex at birth (male/female) | 378/321 | 115/56 | 32/24 | 38/32 |
| Ethnicity/race (%) | ||||
| Asian | 10.60% | 9.41% | 10.71% | 7.14% |
| Black | 11.60% | 18.24% | 19.64% | 15.71% |
| First Nations | 2.01% | 1.76% | 5.36% | 0% |
| Latinx | 5.59% | 5.29% | 0% | 8.57% |
| Middle Eastern | 1.00% | 1.76% | 0% | 2.86% |
| Multiracial | 13.32% | 14.12% | 10.71% | 18.57% |
| NH/PI | 0.29% | 0% | 0% | 0% |
| White | 55.59% | 49.41% | 53.57% | 47.14% |
| Education (y) | 11.43 (3.07) | 11.23 (2.87) | 11.23 (2.12) | 11.62 (2.77) |
| FSIQ | 105.53 (15.92) | 104.87 (17.36) | 106.13 (17.71) | 101.31 (17.94) |
| GFS—Role | 6.21 (2.22) | 5.52 (2.44) | 4.79 (2.51) | 5.59 (2.28) |
| GFS—Social | 6.41 (1.51) | 5.39 (1.50) | 5.16 (1.40) | 5.84 (1.53) |
| CDSS Total | 6.37 (4.48) | 5.53 (4.55) | 7.59 (4.32) | 7.04 (4.57) |
| SOPS—Negative | 12.09 (6.35) | 17.26 (6.13) | 18.59 (4.87) | 14.89 (6.62) |
| SOPS—Positive | 12.99 (3.36) | 13.45 (3.44) | 13.30 (2.78) | 14.14 (3.52) |
| SOPS—Disorganization | 5.19 (3.21) | 6.47 (3.62) | 7.16 (3.45) | 6.96 (3.61) |
| SOPS—General Symptom | 9.42 (4.27) | 8.27 (4.41) | 11.57 (3.81) | 9.96 (4.46) |
Note: Values reflect mean (SD) unless otherwise indicated. CDSS, Calgary Depression Scale for Schizophrenia; DS, deficit syndrome; FSIQ, Full Scale Intelligence Quotient; GFS-Role, Global Functioning Scale—Role; GFS-Social, Global Functioning Scale—Social; NH/PI, Native Hawaiian/Pacific Islander; PNS, persistent negative symptoms; SOPS, Scale of Psychosis Risk Symptoms.
Trajectory Models in Full Sample (Aim 1a)
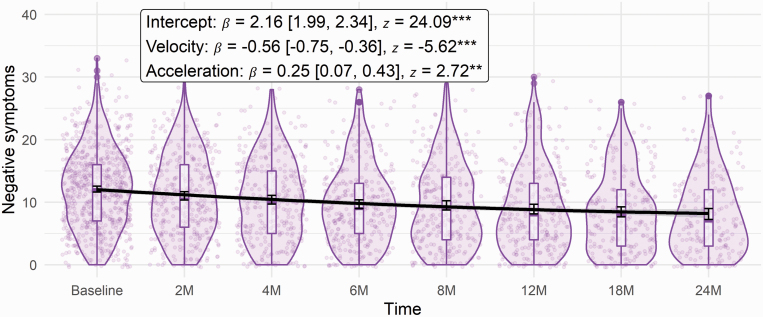
Curvilinear, 2-, and 3-step models showed comparable fit, overall good fit across metrics, and superior fit over the linear model (see table S5, Supplementary Data Content 6, for fit indices of all models). In the linear model, there was a significant downward trajectory of negative symptoms over time (β = −0.737, z = −11.288, P < .001). In the curvilinear model, negative symptoms showed an initial downward trajectory with a shallower (less negative) slope (figure 2). A similar pattern was demonstrated in the 2-step model where negative symptoms decreased significantly from baseline to 6 months (β = −0.673, z = −6.922, P < .001) and 6 to 24 months (β = −0.348, z = −4.174, P < .001); however, the slope became significantly shallower (became less negative) in this second step (β = 0.325, z = 2.321, P = .02). Likewise, the 3-step model showed an initial downward slope (β = −0.724, z = −6.405, P < .001), which then became nonsignificant at the second step (β = −0.126, z = −1.399, P = .162; βdiff = 0.599, zdiff = 3.806, Pdiff < .001), and then decreased at the third step (β = −0.261, z = −3.027, P = .002). The decrease from steps 2 to 3 was nonsignificant (βdiff = −0.136, zdiff = −0.924, Pdiff = .456). See tables S6–S17, Supplementary Data Content 7, for parameter estimates and code of all models.
Fig. 2.
Curvilinear trajectory of negative symptoms in full sample. Note: Error bars reflect 95% confidence intervals around mean for each given time. **P < .01; ***P < .001.
The curvilinear model was adopted for subsequent analyses due to a balance of fit (with some indices showing somewhat worse fit than the 3-step model) and parsimony (as the conclusions among all 3 models are the same). Similarly, the quadratic curvilinear model demonstrated the best fit among mixed-effects models (see table S18, Supplementary Data Content 8). Overall, results supported a significant initial decline in negative symptoms, with the rate of symptom improvement becoming more gradual over time.
Moderators of Trajectory in Full Sample (Aim 1b)
Moderation of growth parameters (intercept, velocity, and acceleration) are presented in table 3. Greater negative symptom intercept was associated with greater positive symptoms, greater depression, and lower social and role functioning. Greater velocity (shallower slope) was associated with greater role functioning and Full Scale Intelligence Quotient (FSIQ) scores. Lower acceleration (fewer deviations in the negative slope) was associated with greater role functioning and FSIQ. In sum, trajectory was only significantly influenced by role functioning and FSIQ. These associations were such that those with greater role functioning and IQ at baseline had slower initial rate of symptom reduction but greater stability of improvements. Mixed-effect model mirrored results (see table S19, Supplementary Data Content 9).
Table 3.
Moderation Estimates of Growth Parameters
| Parameter | Moderator | β [95% CI] | z | P |
|---|---|---|---|---|
| Intercept | Positive symptoms | 0.113 [0.049, 0.178] | 3.438 | .001 |
| Depression | 0.228 [0.166, 0.29] | 7.175 | <.001 | |
| Social functioning | −0.411 [−0.477, −0.346] | −12.271 | <.001 | |
| Role functioning | −0.376 [−0.442, −0.309] | −11.096 | <.001 | |
| FSIQ | 0.016 [−0.05, 0.081] | 0.471 | .638 | |
| Age | −0.017 [−0.08, 0.046] | −0.523 | .601 | |
| Velocity | Positive symptoms | −0.001 [−0.162, 0.159] | −0.013 | .989 |
| Depression | −0.079 [−0.223, 0.065] | −1.078 | .281 | |
| Social functioning | −0.035 [−0.197, 0.127] | −0.425 | .671 | |
| Role functioning | 0.223 [0.069, 0.377] | 2.839 | .005 | |
| FSIQ | 0.211 [0.06, 0.361] | 2.75 | .006 | |
| Age | 0.013 [−0.132, 0.158] | 0.171 | .864 | |
| Acceleration | Positive symptoms | −0.083 [−0.261, 0.094] | −0.92 | .357 |
| Depression | −0.021 [−0.178, 0.136] | −0.264 | .792 | |
| Social functioning | 0.069 [−0.109, 0.248] | 0.764 | .445 | |
| Role functioning | −0.233 [−0.401, −0.065] | −2.716 | .007 | |
| FSIQ | −0.227 [−0.39, −0.064] | −2.722 | .006 | |
| Age | −0.036 [−0.194, 0.122] | −0.452 | .651 |
Note: FSIQ, Full Scale Intelligence Quotient.
Negative Symptom Proxy Subgroup Differences (Aim 2)
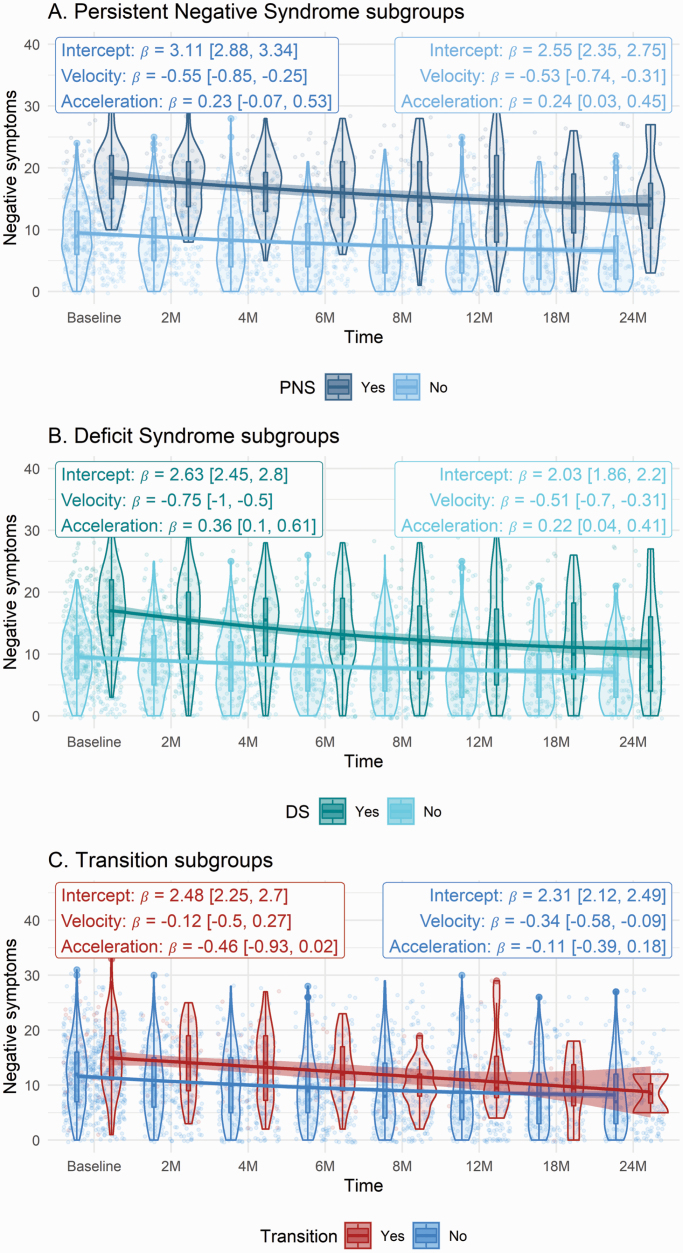
Models exploring moderation by DS and PNS subgroups showed acceptable or good fit, comparable to but somewhat lower than the overall curvilinear model (figure 3). When participants with DS were compared with non-DS, intercept (β = 0.597, z = 19.574, P < .001) was significantly greater, velocity (β = −0.248, z = −3.135, P = .002) was significantly faster (more negative), and acceleration did not significantly differ (β = 0.132, z = 1.465, P = .143). In other words, the DS group at baseline showed elevated negative symptoms which decreased more rapidly overall but nonetheless flattened out over time. In terms of PNS vs non-PNS groups, intercept (β = 0.556, z = 15.736, P < .001) was significantly greater, while velocity (β = −0.025, z = −0.364, P = .716) and acceleration (β = −0.011, z = −0.151, P = .880) did not significantly differ. Thus, the participants who were labeled as having “persistent negative symptoms” started with more severe symptoms but did not demonstrate a different long-term trajectory from the non-PNS group.
Fig. 3.
Negative symptom trajectories of clinical subgroups (a) persistent negative syndrome, (b) deficit syndrome, and (c) transition to psychosis. Note: Error bars reflect 95% confidence intervals around mean for each given time. Estimates within each group are taken from latent growth curve model. DS, deficit syndrome; PNS, persistent negative symptoms.
Transition Status Differences (Aim 3)
Among those who transitioned, intercept (β = 0.173, z = 4.163, P < .001) was significantly greater, velocity (β = 0.22, z = 2.455, P = .014) was significantly shallower (more positive), and acceleration was significantly lower (β = −0.35, z = −3.098, P = .002). Thus, those who transitioned to psychosis showed greater negative symptoms at baseline which decreased less rapidly and for a longer time compared to those who did not transition (figure 3). A model evaluating the prediction of transition by growth trajectory was explored. There were nonsignificant effects of intercept (β = 0.206, z = 1.931, P = .054), velocity (β = −0.418, z = −0.785, P = .432), and acceleration (β = −0.519, z = −0.833, P = .405). The same pattern of results was observed using mixed-effects models. However, the mixed-effect model for differences according to transition status indicated a positive acceleration (reduced decline in symptoms) among those who did not transition (b = 0.06 [0.03, 0.1]) and a negative acceleration (increased decline in symptoms) among those who did (b = −0.17 [−0.33, −0.01]), while the SEM observed negative, nonsignificant accelerations in both groups.
Discussion
The present study characterized the longitudinal trajectory of negative symptoms in CHR participants and determined how that trajectory differs according to PNS, DS, and transition status. The first aim was to evaluate the fit of various statistical models in delineating the longitudinal trajectory of the full NAPLS-3 sample and identify variables that moderate that trajectory. As hypothesized, there was a curvilinear trajectory of negative symptoms, where symptoms showed marked initial improvement within the first 6 months relative to baseline assessment, but the rate of improvement subsequently stabilized or decreased over time (ie, a quadratic trajectory). These findings are inconsistent with prior reports of persistent elevated symptoms15 and negative linear trajectories.14,16,17 Furthermore, the rate and stability of the negative symptom reductions also differed as a function of patient role functioning and IQ. Although CHR participants with greater role functioning and IQ had a slower rate of negative symptom resolution, the stability of negative symptom improvement was greater (ie, the rate of improvement was more sustained). As lower cognition and functioning have been associated with exacerbated negative symptoms,8,14,15 it may also be possible for greater cognition and functioning to serve as protective factors, whereby improved negative symptoms are more resilient to change. Collectively, these findings clarify that the first 6 months after initial ascertainment are a critical window for improving negative symptoms, after which less change can be expected (particularly among those with lower IQ and functioning).
The second aim was to determine whether PNS and DS subgroups displayed different symptom trajectories than CHR participants who did not meet criteria for these categorizations. The current study is the first to demonstrate the utility of delineating via proxy algorithm tool19,22 a DS-CHR subgroup that displays similar demographic and clinical features as schizophrenia samples. Furthermore, this study is the first to test the stability of the DS status by monitoring the trajectory of negative symptoms within this CHR subgroup. As hypothesized, the trajectory of the DS subgroup differed from non-DS, as indicated by higher scores at study ascertainment and a more rapid initial decrease in negative symptoms; however, after this initial difference in the slope of improvement, both groups showed a similar plateau across time. Despite validation of DS classification in CHR at baseline, the longitudinal pattern indicating a significant improvement in negative symptoms among DS-CHR participants suggests that the DS categorization may not be sustained across time. This finding challenges the use of the proxy algorithm that only incorporates cross-sectional symptom data to determine DS group membership, but not longitudinal persistence.
In comparison, the PNS subgroups did not display the same pattern of differences as DS subgroups, further highlighting the utility of each categorization in CHR. Specifically, the PNS group had higher negative symptoms than non-PNS at study ascertainment; however, both groups did not differ in velocity or acceleration parameters (ie, they showed similar rates of initial improvement and subsequent stability). Although the higher baseline scores in PNS vs non-PNS would be expected based on how the groups were defined, the lack of group differences in velocity is not tautological because it would still be possible for PNS to have a steeper improvement than non-PNS since they started higher and could improve more quickly. These findings suggest that additional work may be needed for creating PNS classifications. Prior approaches to defining persistence have incorporated retrospective reports of symptom duration3 and early symptom stability (eg, over initial 6-month period of study ascertainment).15 In the present study’s PNS and non-PNS groups, elevated negative symptoms continued to improve after 6 months in a similar downward curvilinear fashion. Therefore, change from baseline may be a more relevant means of determining PNS than absolute level. Frequent and continuous assessments of negative symptoms in CHR may therefore be necessary to detect gradual improvements in severity that would otherwise go unnoticed with conservative monitoring approaches.
The third aim was to examine differences in the negative symptom trajectory based on transition status. Past research has heavily focused on characterizing the relationship between positive symptoms and psychosis conversion in CHR individuals.16,42,43 The present findings reveal that those who transition have higher negative symptoms at ascertainment and a less steep drop in negative symptoms from months 0 to 6, and this shallow trajectory of symptom improvement persists over a longer time frame (stability). Better long-term monitoring of negative symptoms in CHR individuals may be warranted so the processes associated with limited negative symptom improvement in those with imminent psychosis conversion can be detected and treated. This recommendation is important when psychosis patients tend to have relatively less insight and help-seeking for their negative symptoms compared to positive symptoms.44 The experience of positive symptoms may dominate patient reported concerns, which may then lead clinicians to de-prioritize rapid and sustainable remission of negative symptoms.
The overall trend of a downward, curvilinear trajectory of negative symptoms in the full sample and clinical subgroups was a novel and unexpected finding. There are a few possible explanations to consider. First, it is possible that negative symptom presentation is more dynamic and transitory for those at CHR compared to individuals with a more chronic course of schizophrenia. This perspective further highlights the need to assess clinical subgroups like DS and PNS in future CHR samples based on longitudinal symptom patterns relative to a baseline anchor point vs cross-sectional or absolute cutoff values. Second, findings may be reflective of a regression to the mean phenomenon to which most help-seeking, clinical groups are vulnerable. Comparison of observed negative symptom trajectories in CHR participants receiving treatment vs no treatment may help rule out which trajectory characteristics may be attributable to regression to the mean. Third, it is possible that a decrease in negative symptoms resulted from the social enrichment that accompanies study participation. Future research looking to isolate the speculated mechanisms of the observed curvilinear negative symptom trajectory may benefit from ecological momentary assessment methods that introduce minimal assessor-participant interaction benefits.
A strength of this study is its rich dataset of multiple assessments over a 24-month period and across multiple sites, which afforded the unique opportunity to model the negative syndrome trajectory exhibited by CHR patients. However, the results should be interpreted considering study limitations. First, the “baseline” assessment of negative symptoms in this study is not guaranteed to be an evaluation at symptom onset. Rather, baseline scores are a snapshot of symptoms upon study enrollment, thereby somewhat complicating interpretation of symptom trajectories. The trajectories captured by this study may have measured different portions of the overall symptom progression across participants. An unreliable baseline assessment point and its interpretation challenges appears to be a pervasive limitation within the symptom trajectory literature. Although the onset of positive symptoms is often recorded, studies struggle to have young participants accurately define the onset of negative symptoms and the NAPLS-3 study was no exception to this challenge. Present study findings may still be interpreted as longitudinal negative symptom dynamics in CHR anchored by an early point in emerging symptom trajectories, which previously has not been well established. Second, besides a 1-year post-transition assessment, further follow-ups in NAPLS-3 were discontinued once a participant made the transition to psychosis. The negative symptom trajectory of those who make a transition to psychosis may be better characterized with more frequent follow-ups. Third, all SOPS negative symptoms were used, despite some studies suggesting the exclusion of the N6 Occupational Functioning item,15,45 which may measure functional capacity in addition to negative symptoms. Our analysis retained this item based on improved internal consistency. Lastly, it is possible that results reflect the unique characteristics of the current study sample (ie, CHR individuals who are motivated to participate in all follow-up assessments of a longitudinal study) and may not be generalizable to all CHR individuals.
Despite these limitations, findings clarify that the first 6 months after an established baseline may be a critical window for improving negative symptoms. Less improvement is likely after this period. As such, it would be advantageous to direct monitoring and intervention efforts to this early period. Methods for reducing heterogeneity in negative symptoms (eg, DS and PNS proxy algorithms) may inform trajectories and treatment planning; however, further psychometric investigation is needed to optimize these tools in CHR individuals before they become viable for use in clinical practice.
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Contributor Information
Tanya Tran, Department of Psychology, Queen’s University, Kingston, ON, Canada.
Michael J Spilka, Department of Psychology, University of Georgia, Athens, GA, USA.
Ian M Raugh, Department of Psychology, University of Georgia, Athens, GA, USA.
Gregory P Strauss, Department of Psychology, University of Georgia, Athens, GA, USA.
Carrie E Bearden, Department of Psychiatry and Biobehavioral Sciences, UCLA, Los Angeles, CA, USA.
Kristin S Cadenhead, Department of Psychiatry, UCSD, San Diego, CA, USA.
Tyrone D Cannon, Department of Psychology, Yale University, New Haven, CT, USA; Department of Psychiatry, Yale University, New Haven, CT, USA.
Barbara A Cornblatt, Department of Psychiatry, Zucker Hillside Hospital, Long Island, NY, USA.
Matcheri Keshavan, Department of Psychiatry, Harvard Medical School at Beth Israel Deaconess Medical Center and Massachusetts Mental Health Center, Boston, MA, USA.
Daniel H Mathalon, Department of Psychiatry, UCSF, and SFVA Medical Center, San Francisco, CA, USA.
Thomas H McGlashan, Department of Psychiatry, Yale University, New Haven, CT, USA.
Diana O Perkins, Department of Psychiatry, University of North Carolina, Chapel Hill, NC, USA.
Larry J Seidman, Department of Psychiatry, Harvard Medical School at Beth Israel Deaconess Medical Center and Massachusetts Mental Health Center, Boston, MA, USA.
William S Stone, Department of Psychiatry, Harvard Medical School at Beth Israel Deaconess Medical Center and Massachusetts Mental Health Center, Boston, MA, USA.
Ming T Tsuang, Department of Psychiatry, UCSD, San Diego, CA, USA; Institute of Genomic Medicine, University of California, La Jolla, CA, USA.
Elaine F Walker, Department of Psychology, Emory University, Atlanta, GA, USA; Department of Psychiatry, Emory University, Atlanta, GA, USA.
Scott W Woods, Department of Psychiatry, Yale University, New Haven, CT, USA.
Jean M Addington, Department of Psychiatry, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada.
Funding
This study was supported by the National Institute of Mental Health (grant U01MH081984 to Dr Addington; grant U01MH081928 to Dr Stone; grant U01MH081944 to Dr Cadenhead; grant U01MH081902 to Drs Cannon and Bearden; grant U01MH082004 to Dr Perkins; grant U01MH081988 to Dr Walker; grant U01MH082022 to Dr Woods; grant U01MH076989 to Dr Mathalon; grant UO1MH081857 to Dr Cornblatt).
Author Contributions
The study was designed by Drs Addington, Cannon, Cornblatt, Cadenhead, Woods, McGlashan, Perkins, Tsuang, Bearden, Walker, Mathalon, and Seidman. Mr Raugh and Dr Spilka undertook data analysis. Dr Tran wrote the initial manuscript. Drs Addington, Strauss, Spilka, and Tran were involved in writing subsequent drafts of the manuscript. All authors contributed to and approved the final manuscript.
References
- 1.Lencz T, Smith CW, Auther A, Correll CU, Cornblatt B.. Nonspecific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophr Res. 2004;68:37–48. [DOI] [PubMed] [Google Scholar]
- 2.Piskulic D, Addington J, Cadenhead KS, et al. . Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Res. 2012;196:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrión RE, Demmin D, Auther AM, et al. . Duration of attenuated positive and negative symptoms in individuals at clinical high risk: associations with risk of conversion to psychosis and functional outcome. J Psychiatr Res. 2016;81:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brucato G, Masucci MD, Arndt LY, et al. . Baseline demographics, clinical features and predictors of conversion among 200 individuals in a longitudinal prospective psychosis-risk cohort. Psychol Med. 2017;47:1923–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corcoran CM, Kimhy D, Parrilla-Escobar MA, et al. . The relationship of social function to depressive and negative symptoms in individuals at clinical high risk for psychosis. Psychol Med. 2011;41:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demjaha A, Valmaggia L, Stahl D, Byrne M, McGuire P.. Disorganization/cognitive and negative symptom dimensions in the at-risk mental state predict subsequent transition to psychosis. Schizophr Bull. 2012;38:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Healey KM, Penn DL, Perkins D, Woods SW, Keefe RS, Addington J.. Latent profile analysis and conversion to psychosis: characterizing subgroups to enhance risk prediction. Schizophr Bull. 2018;44:286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valmaggia LR, Stahl D, Yung AR, et al. . Negative psychotic symptoms and impaired role functioning predict transition outcomes in the at-risk mental state: a latent class cluster analysis study. Psychol Med. 2013;43:2311–2325. [DOI] [PubMed] [Google Scholar]
- 9.Velthorst E, Nieman DH, Becker HE, et al. . Baseline differences in clinical symptomatology between ultra high risk subjects with and without a transition to psychosis. Schizophr Res. 2009;109:60–65. [DOI] [PubMed] [Google Scholar]
- 10.Werbeloff N, Dohrenwend BP, Yoffe R, van Os J, Davidson M, Weiser M.. The association between negative symptoms, psychotic experiences and later schizophrenia: a population based longitudinal study. PLoS One. 2015;10:e0119852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang TH, Tang XC, Li, HJ, et al. . Clinical subtypes that predict conversion to psychosis: a canonical correlation analysis study from the ShangHai At Risk for Psychosis program. Aust N Z J Psychiatry. 2020;54:482–495. [DOI] [PubMed] [Google Scholar]
- 12.Devoe DJ, Peterson A, Addington J.. Negative symptom interventions in youth at risk of psychosis: a systematic review and network meta-analysis. Schizophr Bull. 2018;44:807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauss GP, Pelletier-Baldelli A, Visser KF, Walker EF, Mittal VA.. A review of negative symptom assessment strategies in youth at clinical high-risk for psychosis. Schizophr Res. 2020;222:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann JA, Schmidt SJ, McGorry PD, et al. . Trajectories of symptom severity and functioning over a three-year period in a psychosis high-risk sample: a secondary analysis of the Neurapro trial. Behav Res Ther. 2020;124:103527. [DOI] [PubMed] [Google Scholar]
- 15.Devoe DJ, Lu L, Cannon TD, et al. . Persistent negative symptoms in youth at clinical high risk for psychosis: a longitudinal study. Schizophr Res. 2021;227:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hengartner MP, Heekeren K, Dvorsky D, Walitza S, Rössler W, Theodoridou A.. Course of psychotic symptoms, depression and global functioning in persons at clinical high risk of psychosis: results of a longitudinal observation study over three years focusing on both converters and non-converters. Schizophr Res. 2017;189:19–26. [DOI] [PubMed] [Google Scholar]
- 17.Schlosser DA, Fisher M, Gard D, Fulford D, Loewy RL, Vinogradov S.. Motivational deficits in individuals at-risk for psychosis and across the course of schizophrenia. Schizophr Res. 2014;158:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velthorst E, Nieman DH, Klaassen RMC, et al. . Three-year course of clinical symptomatology in young people at ultra high risk for transition to psychosis. Acta Psychiatr Scand. 2011;123:36–42. [DOI] [PubMed] [Google Scholar]
- 19.Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT.. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58:165–171. [DOI] [PubMed] [Google Scholar]
- 20.Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galderisi S, Mucci A, Bitter I, et al. ; Eufest Study Group. Persistent negative symptoms in first episode patients with schizophrenia: results from the European First Episode Schizophrenia Trial. Eur Neuropsychopharmacol. 2013;23:196–204. [DOI] [PubMed] [Google Scholar]
- 22.Kirkpatrick B, Buchanan RW, Breier A, Carpenter WT Jr. Case identification and stability of the deficit syndrome of schizophrenia. Psychiatry Res. 1993;47:47–56. [DOI] [PubMed] [Google Scholar]
- 23.Mucci A, Merlotti E, Üçok A, Aleman A, Galderisi S.. Primary and persistent negative symptoms: concepts, assessments and neurological bases. Schizophr Res. 2017;186:19–28. [DOI] [PubMed] [Google Scholar]
- 24.López-Díaz A, Lara I, Lahera G.. Is the prevalence of the deficit syndrome in schizophrenia higher than estimated? Results of a meta-analysis. Psychiatry Investig. 2018;15:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirkpatrick B, Mucci A, Galderisi S.. Primary, enduring negative symptoms: an update on research. Schizophr Bull. 2017;43:730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirschner M, Aleman A, Kaiser S.. Secondary negative symptoms—a review of mechanisms, assessment and treatment. Schizophr Res. 2017;186:29–38. [DOI] [PubMed] [Google Scholar]
- 27.Azar M, Pruessner M, Baer LH, Iyer S, Mall AK, Lepage M.. A study on negative and depressive symptom prevalence in individuals at ultra-high risk for psychosis. Early Interv Psychiatry. 2018;12:900–906. [DOI] [PubMed] [Google Scholar]
- 28.Galderisi S, Mucci A, Buchanan RW, Arango C.. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. 2018;5:664–677. [DOI] [PubMed] [Google Scholar]
- 29.Addington J, Liu L, Brummitt K, et al. . North American Prodrome Longitudinal Study (NAPLS 3): methods and baseline description. Schizophr Res. 2022;243:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGlashan T, Walsh B, Woods S.. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-Up. New York, NY: Oxford University Press; 2010. [Google Scholar]
- 31.Miller TJ, McGlashan TH, Rosen JL, et al. . Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. [DOI] [PubMed] [Google Scholar]
- 32.Addington D, Addington J, Maticka-Tyndale E.. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry. 1993;163:39–44. [PubMed] [Google Scholar]
- 33.Auther AM, Smith CW, Cornblatt BJ.. Global Functioning: Social Scale (GF: Social). Glen Oaks, NY: Zucker-Hillside Hospital; 2006. [Google Scholar]
- 34.Cornblatt BA, Auther AM, Niendam T, et al. . Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33:688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goetz RR, Corcoran C, Yale S, et al. . Validity of a ‘proxy’ for the deficit syndrome derived from the Positive and Negative Syndrome Scale (PANSS). Schizophr Res. 2007;93:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu CY.Evaluating Cutoff Criteria of Model Fit Indices for Latent Variable Models with Binary and Continuous Outcomes. Los Angeles, CA: University of California; 2002. [Google Scholar]
- 37.Akaike H. Factor analysis and AIC. In: Parzen E, Tanabe K, Kitagawa G, eds. Selected Papers of Hirotugu Akaike. New York, NY: Springer; 1987:371–386. [Google Scholar]
- 38.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. [DOI] [PubMed] [Google Scholar]
- 39.Tucker LR, Lewis C.. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38:1–10. [Google Scholar]
- 40.Steiger JH. Structural model evaluation and modification: an interval estimation approach. Multivariate Behav Res. 1990;25:173–180. [DOI] [PubMed] [Google Scholar]
- 41.McNeish D, Matta T.. Differentiating between mixed-effects and latent-curve approaches to growth modeling. Behav Res Methods. 2018;50:1398–1414. [DOI] [PubMed] [Google Scholar]
- 42.DeVylder JE, Muchomba FM, Gill KE, et al. . Symptom trajectories and psychosis onset in a clinical high-risk cohort: the relevance of subthreshold thought disorder. Schizophr Res. 2014;159:278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang T, Xu L, Chen Y, et al. . Conversion to psychosis in adolescents and adults: similar proportions, different predictors. Psychol Med. 2021;51:2003–2011. [DOI] [PubMed] [Google Scholar]
- 44.Carbon M, Correll CU.. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 2014;19:35–53. [DOI] [PubMed] [Google Scholar]
- 45.Meyer EC, Carrión RE, Cornblatt BA, et al. ; NAPLS group. The relationship of neurocognition and negative symptoms to social and role functioning over time in individuals at clinical high risk in the first phase of the North American Prodrome Longitudinal Study. Schizophr Bull. 2014;40:1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.