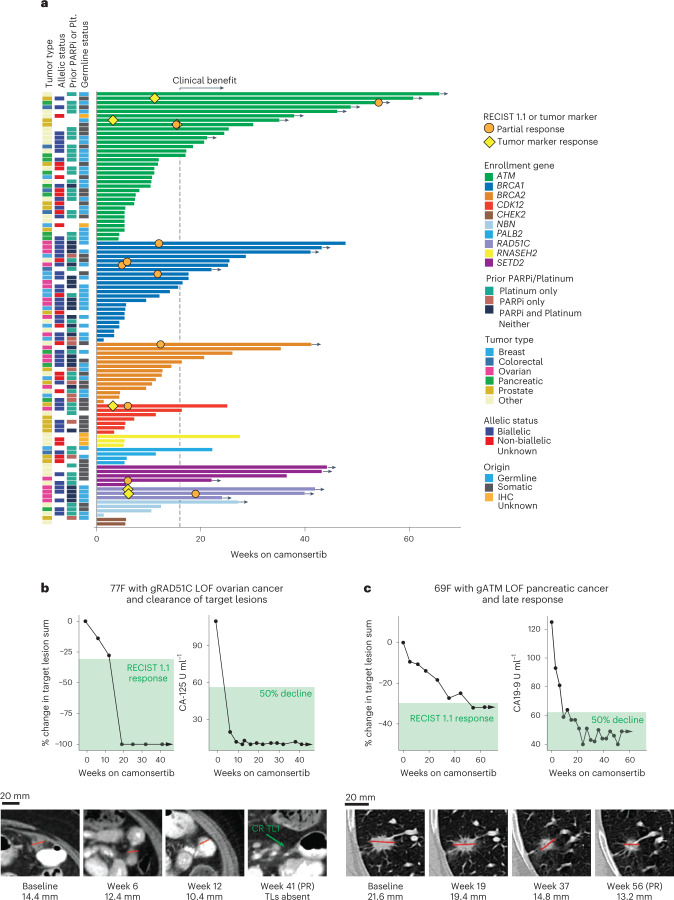
Fig. 2. Clinical outcomes in TRESR.
a, Duration of treatment by genotype. Clinical benefit is defined as a treatment duration of at least 16 weeks (without evidence of progression) and/or a RECIST 1.1 or tumor marker response. The gray dotted line indicates 16 weeks. b, Case report for a patient (n = 1) with gRAD51C LOF ovarian cancer who had complete disappearance of the TLs. c, Case report for a patient (n = 1) with a gATM LOF pancreatic cancer who had a late response to camonsertib. 69F, 69-year-old female; 77F, 77-year-old female; g, genomic; Plt., platinum.