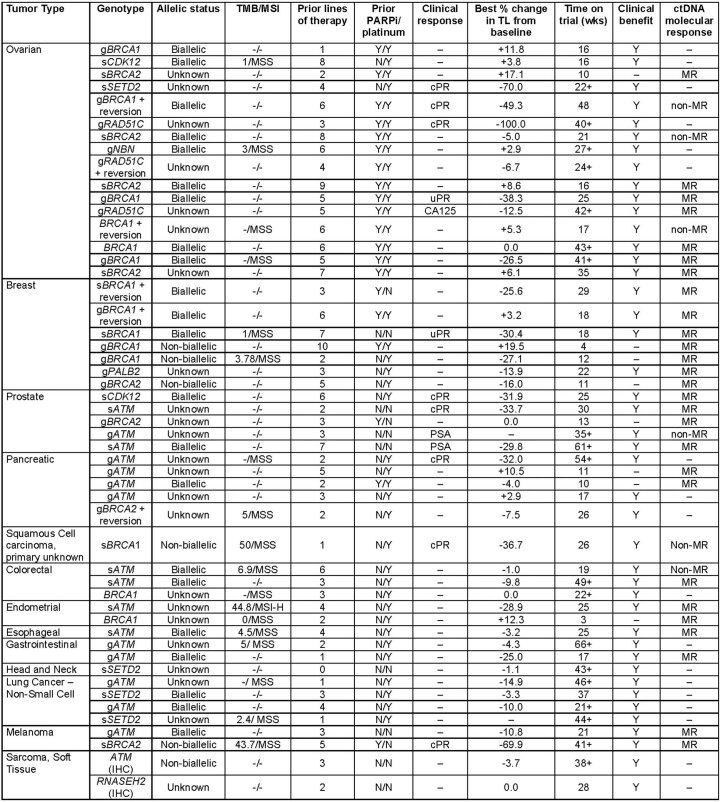
Extended Data Table 4.
Summary of clinical and molecular characteristics of patients with clinical response, clinical benefit and MR
Data cutoff date was 22 March 2022. ‘+’ indicates that the treatment is still ongoing. Clinical benefit defined as having RECIST 1.1/tumor marker response or time on treatment ≥16 weeks without PD. g, germline; IHC, immunohistochemistry; MR, molecular response; MSI, microsatellite instability; MSI-H, microsatellite instability high; MSS, microsatellite stable; N, no; PARPi, poly adenosine diphosphate-ribose polymerase inhibitor; PSA, prostate specific antigen; s, somatic; TL, target lesion; TMB; tumor mutation burden; uPR, unconfirmed partial response; wks, weeks; Y, yes.