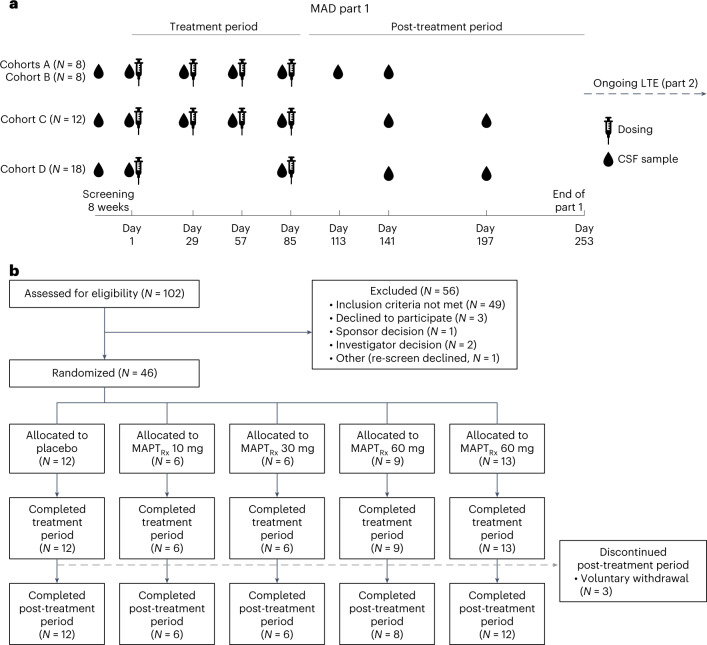
Fig. 1. Trial design and patient flow diagram.
a, Dosing and CSF sample collection for MAD part 1. CSF samples were obtained before the administration of study drug on days 1, 29, 57 and 85 for cohort A (10 mg MAPTRx or placebo monthly), cohort B (30 mg MAPTRx or placebo monthly) and cohort C (60 mg MAPTRx or placebo monthly) and on days 1 and 85 for cohort D (115 mg MAPTRx or placebo quarterly). The results of CSF samples obtained during screening and on day 1 (baseline) were averaged to serve as the baseline assessment, and the CSF samples on days 29, 57 and 85 served as 28 day, 56 day or 84 day post-dose trough samples. Two CSF samples were obtained in the post-treatment period, on either day 113 or day 141 for cohorts A and B and day 141 and day 197 for cohorts C and D. b, Patient flow during MAD part 1. Eligible patients were randomly assigned in a 3:1 ratio to receive the ASO MAPTRx or placebo in all cohorts.