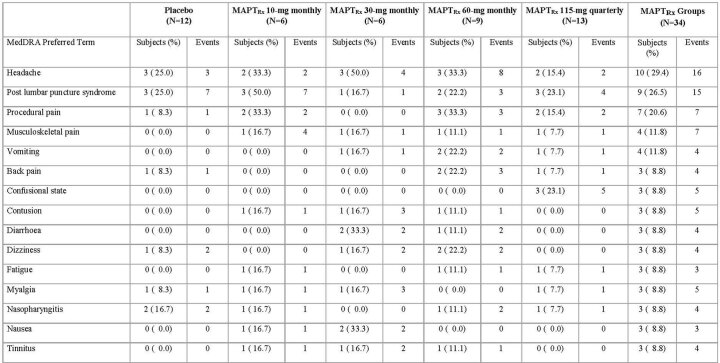
Extended Data Table 1.
Adverse Events Reported in at Least Three Patients* Receiving MAPTRx According to Treatment Group
*Patients reporting more than one adverse event were counted only once for the incidence using the most severe grade and, if there was a missing severity for the same subject, then the non-missing severity, if available, was chosen for the same subject.
**All headache events including those related to lumbar puncture procedure.