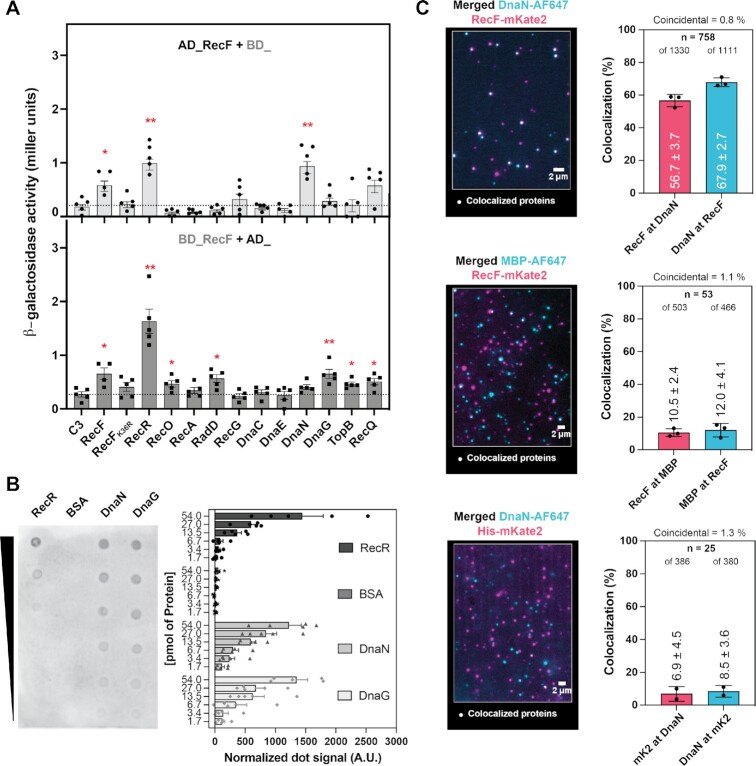
Figure 8.
RecF interacts with β-clamp and DnaG. The interaction between RecF and partners was tested in vivo by yeast-two hybrid (A) and in vitro by far western blot (B) or by single-molecule interaction assay (C). (A) Combination of yeast transformed with RecF fused to either domain activator (AD) upper panel or binding domains (BD) lower panel and the control C3 or the indicated protein, involved either in DNA repair (RecF, RecFK36R, RecR, RecO, RecA, RadD, RecG, TopB, RecQ) or DNA replication (DnaC, DnaE, DnaN, DnaG), fused to the other domain were used. The strength of the interactions was tested by beta-galactosidase activity. A series of four or five biological replicates were carried out for each combination. (B) Far western blot using RecF as prey and RecR, BSA, DnaN and DnaG as baits. Increasing concentration from bottom to top of baits were spotted on the nitrocellulose membrane. Membrane was incubated with 0.2 μM of RecF before washing, incubation with primary and secondary antibodies to reveal the interaction partners. The interaction with partners was quantified as described in the method section. The average and s.e.m. of four biological replicates were plotted for each protein, dot represent individual result of each experiment. (C) Single-molecule interaction assay of fluorescently labelled proteins was used to analyze the colocalization of RecFmKate2 and DnaN_AF647 with partners. Merged channels of the dual color imaging of the protein and colocalization analysis histograms. The number of colocalized foci n and the total number of proteins are indicated for each condition. The coincidental colocalization (random) and the experimental percentage of colocalization of one protein to another are indicated in the histograms.