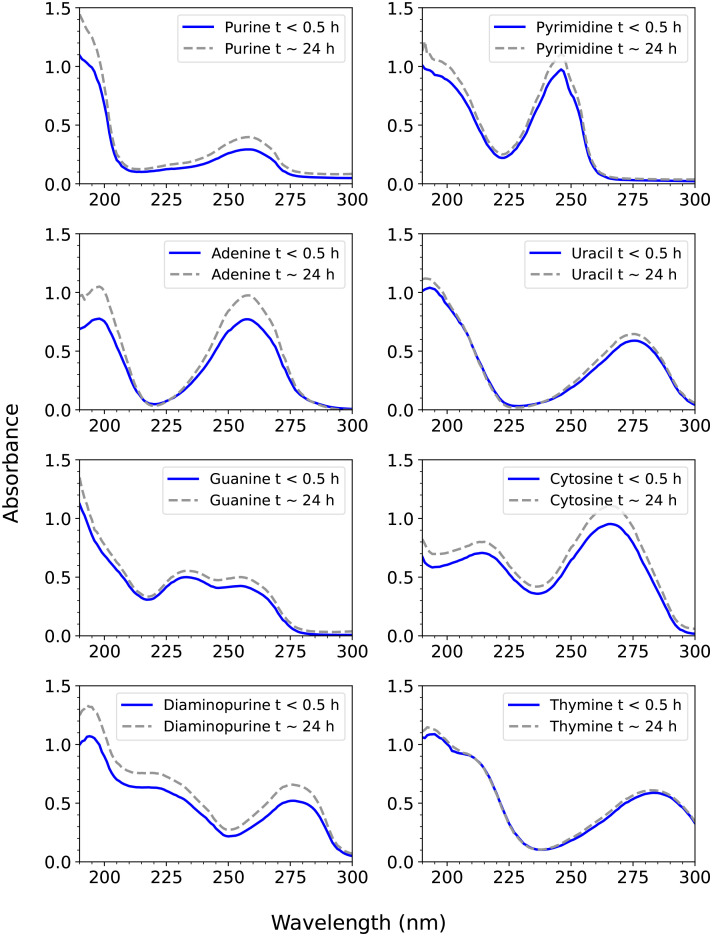
Fig. 2.
UV spectroscopy of the eight compounds studied in 98% w/w sulfuric acid. The absorbance, defined as A = εLc, where A is absorbance (dimensionless), ε is the molar absorption coefficient (in units of M−1 cm1), L is pathlength (in cm), and c is concentration (in units of M), as a function of wavelength. Each compound shows two characteristic UV peaks, due to π-π conjugated bonds. The blue line shows the UV spectrum measured within about 15 to 20 min after mixing of the compound in 98% w/w H2SO4 in H2O and the gray dashed line is the same compound measured after about 24 h. While some compounds have a higher absorbance due to more dissolution over the 24 h, the same peak wavelength maximum and peak shape demonstrates stability of each compound in 98% w/w sulfuric acid.