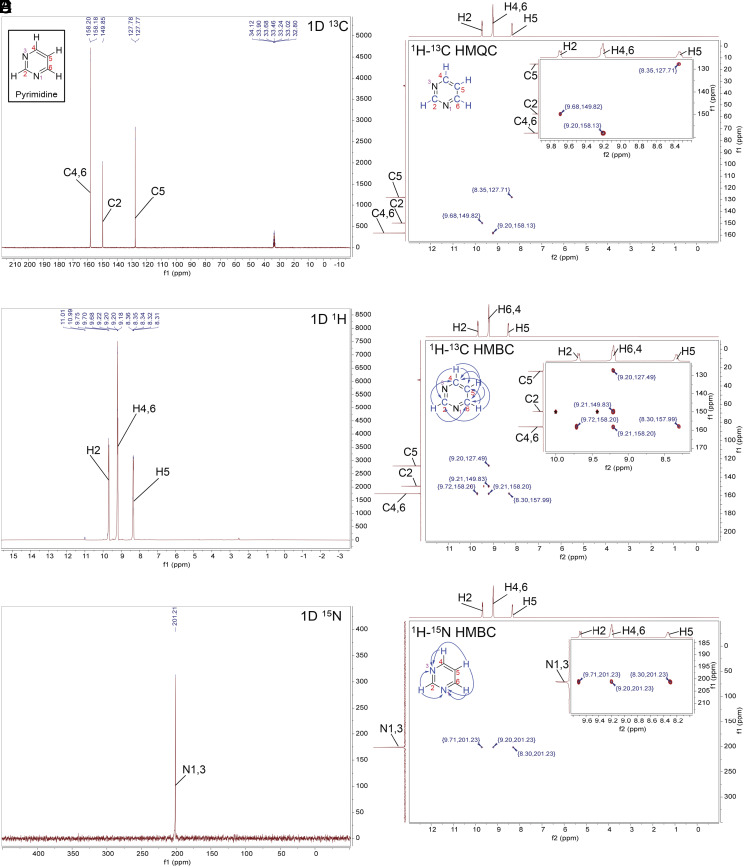
Fig. 6.
NMR spectra for pyrimidine in concentrated sulfuric acid (98% D2SO4 and 2% D2O, by weight, with reference DMSO-d6) at room temperature. The NMR experiments confirm the stability of pyrimidine in concentrated sulfuric acid. (A) 1D 13C NMR. (B) 1D 1H NMR. The solvent peak is suppressed for clarity. (C) 1D 15N NMR. (D) The 2D 1H-13C HMQC NMR shows direct bonding between H and C atoms in the pyrimidine ring structure. (E) The 2D 1H-13C HMBC NMR shows signals that correspond to hydrogen and carbon atoms separated from each other by the distance of 2 or 3 chemical bonds in the pyrimidine ring structure (blue arrows). The HMBC one-bond artifacts are marked with an asterisk (*). (F) The 2D 1H-15N HMBC NMR shows 2 or 3 bond distances between hydrogens attached to carbon and nitrogen atoms (blue arrows).