Fig. 1.
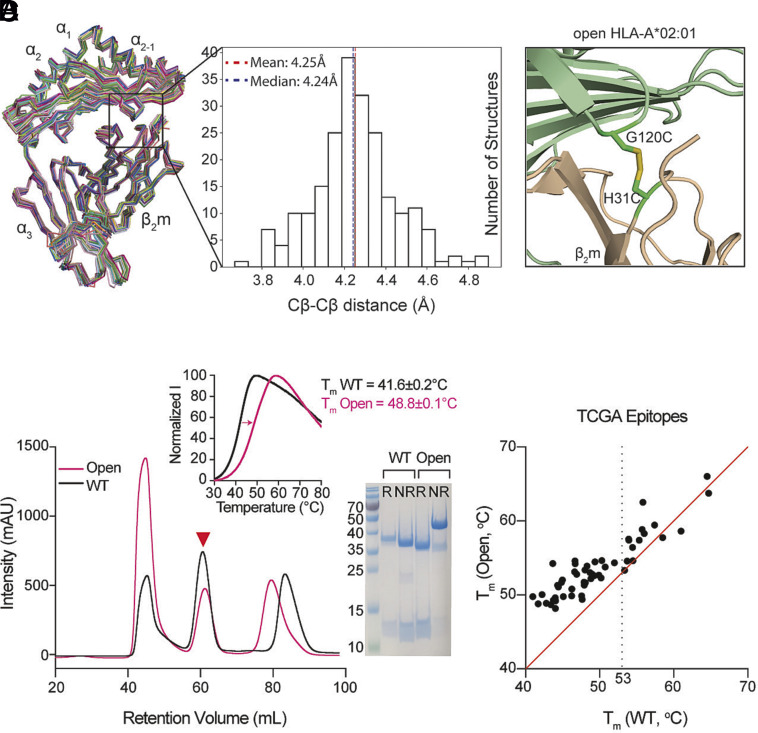
Structure-guided stabilization of suboptimal peptide-loaded HLA-A*02:01 by engineered disulfide between the HC and β2m. (A) Structure alignment and distribution of Cβ–Cβ distances between positions G120 of the HC and H31 of the β2m derived from 215 pMHC-I/β2m cocrystal structures with resolution values ≤3 Å. The structures of 52 distinct alleles are aligned by Cα atoms of α1, α2, and α3 domains as ribbons. (B) Structural model of HLA-A*02:01/β2m/TAX9 (PDB ID:1DUZ) with G120 and H31 mutated to cysteines. HLA-A*02:01 HC was colored in light green and β2m in wheat. (C) SEC traces of the WT (black) and the G120C/H31C open (pink) HLA-A*02:01/β2m/TAX8. The red triangle arrowhead indicates the complex peaks and is further confirmed by SDS/PAGE analysis in reduced (R) or nonreduced (NR) conditions. DSF shows thermal stability curves of the WT in black (Tm = 41.6 °C) and the open variant in pink (Tm = 48.8 °C). The average of three technical replicates (mean) is plotted. (D) Thermal stability correlation of the WT and open HLA-A*02:01/TAX8 loaded with each of 50 peptides from the Cancer Genome Atlas (TCGA) epitope library is shown in dots. The average of three technical replicates (mean) is plotted. The red line represents a conceptual 1:1 correlation (no difference in thermal stabilities).