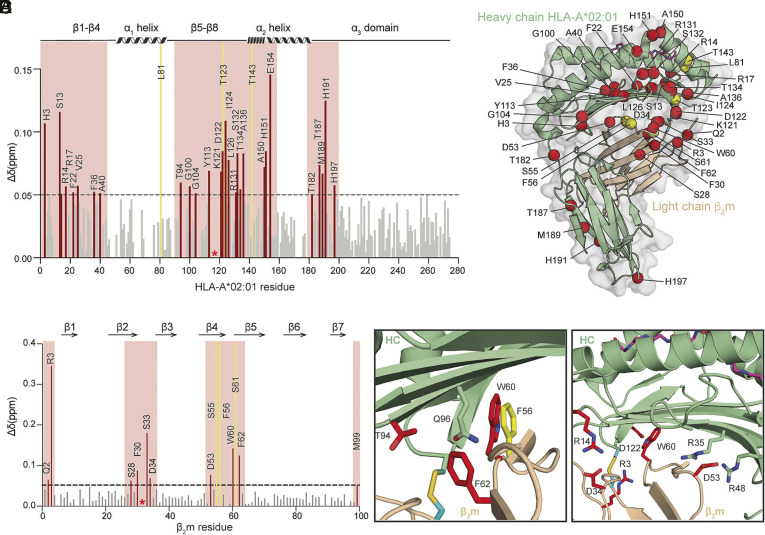
Fig. 2.
Disulfide-engineered pHLA-A*02:01 shows induced conformational adaptations in solution. (A and B) Calculated CSPs between the WT and open HLA-A*02:01/β2m/MART1 are plotted as bar graphs across (A) HC and (B) β2m amide backbone. A significance threshold of 0.05 ppm is determined that is fivefold higher than the 1H sensitivity of the NMR instrument. Residues with significant CSPs are highlighted in red, and exchange-broadened residues in the open HLA-A*02:01 relative to the WT are colored in yellow. Cysteine mutations (G120C and H31C) are indicated by a red asterisk. (C) Residues with CSPs above the significance threshold and exchange broadened in the open HLA-A*02:01/β2m/MART1 are plotted as red and yellow spheres for the amide, respectively, on a representative HLA-A*02:01/β2m/MART1 crystal structure (PDB ID: 3MRQ). (D and E) Enlarged images of (D) hydrophobic residues near the disulfide bond and (E) residues within the hydrogen network. Side chains are displayed and highlighted in red for significant CSPs and yellow for exchange broadening.