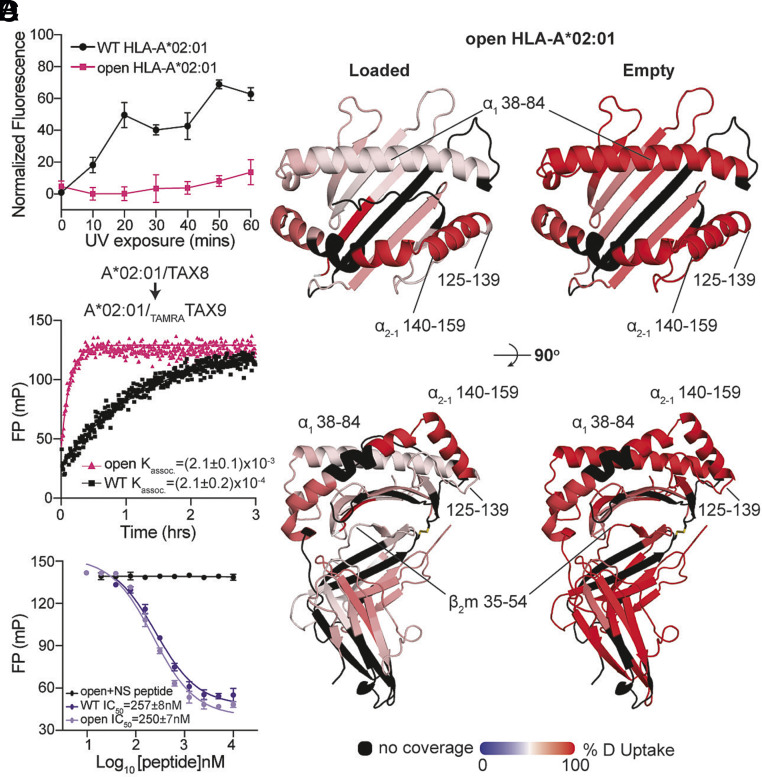
Fig. 3.
Engineered disulfide stabilizes MHC-I at an open, peptide-receptive conformation. (A) Normalized fluorescence measured at 25 °C for the WT or open HLA-A*02:01/KILGFVFJV upon UV exposure. The duration of UV irradiation is shown on the x axis. Results of three technical replicates (mean ± σ) are plotted. (B) Deuterium uptake resolved to individual peptide fragments upon 600-second deuterium labeling for peptide-loaded (Left) and empty (Right) states of open HLA-A*02:01 is mapped onto the WT HLA-A*02:01 crystal structure (PDB ID: 1DUZ) for visualization. Red- and blue-colored regions indicate segments containing peptides with 100% ΔHDX (red—more deuteration) or 0% ΔHDX (blue—less deuteration), respectively; black indicates regions where peptides were not obtained for peptide-loaded and empty protein states. (C) Association profiles of the fluorophore-conjugated peptide TAMRATAX9 to the WT or open HLA-A*02:01/TAX8, as indicated. Results of three replicates (mean) are plotted. (D) Competitive binding of TAMRATAX9 to the WT or open HLA-A*02:01/TAX8 as a function of increasing peptide concentration, measured by fluorescence polarization. An irrelevant peptide, p29 (YPNVNIHNF), was used as a negative control.