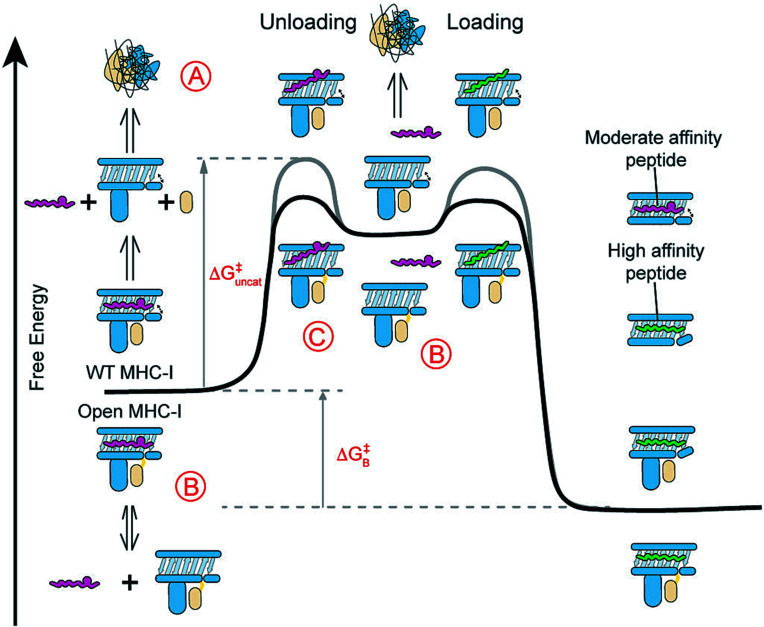
Fig. 6.
Open MHC-I molecules modulate the free energy landscape of peptide exchange. (A) WT MHC-I molecules loaded with moderate-affinity placeholder peptides can spontaneously generate empty molecules via peptide unloading, leading to β2m loss and irreversible protein aggregation. The incoming high-affinity peptides rescue these empty molecules, resulting in stable ternary complexes. The binding free energy (ΔGB) of the moderate-affinity peptide exchanged for the high-affinity peptide is unchanged between the open and WT molecules. (B and C) Open MHC-I enhances peptide exchange by (B) stabilizing empty molecules to prevent their aggregation and (C) lowering the activation free energy (ΔGuncat) for peptide unloading via a stabilized open conformation. The gray and black lines indicate the free energy curves for the WT and open MHC-I, respectively.