Figure 8.
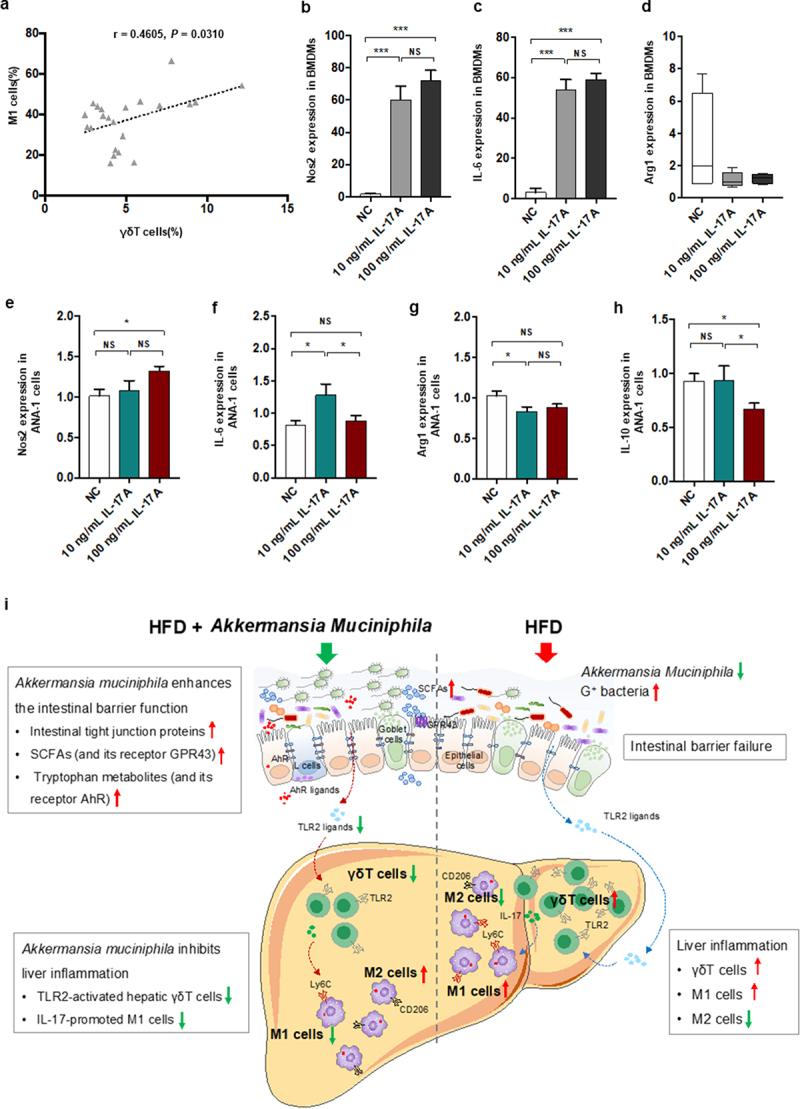
IL − 17 signals promoted macrophage polarization into proinflammatory M1 cells. (a) the relationship between hepatic γδT cells and M1 cells in HFD-induced NASH mice. n = 24 mice. Gene expression of Nos −1 (b), IL − 6 (c) and Arg −1 (d) in bone marrow-derived macrophages (BMDMs) after 10 or 100 ng/mL IL − 17 stimulation. Gene expression of Nos −1 (e), IL − 6 (f), Arg −1 (g) and IL − 10 (h) in Ana −1 cells after 10 or 100 ng/mL IL − 17 stimulation. Data are shown as the mean ± SEM or the median with interquartile range. p values were determined using one-way ANOVA or the Kruskal‒Wallis test. *p < 0.05, ** p < 0.01, *** p < 0.001. (i) Schematic showing the regulatory role of Akkermansia muciniphila in HFD-induced hepatic inflammation. Akkermansia muciniphila enriches intestinal tight junction proteins, SCFAs (and their receptor GPR43), and tryptophan metabolites (and their receptor AhR) and further enhances intestinal barrier function. Following the restoration of intestinal barrier function, Akkermansia muciniphila inhibits liver inflammation by downregulating gut-derived signals (TLR2) in the liver. Specifically, hepatic γδT cells activated by molecules of G+ bacteria (TLR2 ligands) were decreased in HFD mice. Moreover, Akkermansia muciniphila reduced IL − 17-promoted hepatic proinflammatory macrophages (M1 cells).
