Abstract
Background:
Alcohol stimulates cerebral blood flow (CBF) in brain reward regions, but neural processes that support sustained alcohol motivation after the first drink are not well understood.
Methods:
Using a novel placebo-controlled, randomized, crossover experiment, 27 alcohol users who binge drink (BD; 15 M, 12 F) and 25 social drinkers (SD; 15 M, 10 F) who do not binge, engaged in a behavioral test of self-motivated alcohol consumption to assess initial alcohol motivation using an Alcohol Taste Test (ATT) conducted with alcoholic and non-alcoholic placebo beer on separate days immediately prior to perfusion functional magnetic resonance imaging (fMRI) scanning. Immediately following each fMRI scan, participants also engaged in a post-scan ATT with placebo beer on both days to assess sustained alcohol self-motivation without active alcohol effects and relative to initial alcohol self-motivation. Linear mixed-effects models were used to examine the effects of drinking group on the placebo-controlled effect of initial alcohol motivation on brain perfusion (whole brain corrected p<0.001, cluster corrected p<0.025) and on the relationship between placebo-controlled brain perfusion and sustained alcohol motivation.
Results:
Initial alcohol self-motivation in the alcohol relative to placebo session led to markedly decreased activation in the medial orbitofrontal cortex (OFC) and the ventral striatum, indicative of neural reward tolerance, but also enhanced neural response in behavioral intention regions of the supplementary motor area (SMA) and inferior frontal gyrus (IFG) regions in the BD relative to SD group. Moreover, greater sustained alcohol motivation was seen in BD relative to SD in the post-scan ATT in the alcohol relative to placebo session. Correspondingly, only in BD and only in the alcohol session, lower alcohol-induced OFC response correlated with concurrent sensitized SMA response, and each predicted the subsequent sustained higher alcohol motivation in the post-scan ATT.
Conclusions:
The findings suggest that alcohol-related OFC tolerance plays a significant role in sustained alcohol motivation, and that both specific alcohol-related neural reward tolerance and pre-motor sensitization responses may contribute to escalating alcohol motivation to drive excessive alcohol intake, even prior to development of alcohol use disorders.
Keywords: fMRI, orbitofrontal cortex, reward, binge drinking, motivation
Introduction
Enjoying an alcoholic drink or two socially is common. But why do some people want more? Worldwide rises in binge drinking have increased the risk of alcohol-related negative health consequences, including the development of alcohol use disorder (AUD) (Knox et al., 2019, SAMHSA, 2020). However, mechanisms driving sustained alcohol motivation after initial intake are not well-understood. The subjective and physiologically stimulating effects of alcohol are well known, as is the concomitant activation of the nucleus accumbens/ventral striatum (VS), one of the regions involved in signaling alcohol reward (Burke and Tobler, 2016, Blaine et al., 2016, Boileau et al., 2003, Leyton et al., 2002). Binge alcohol intake is also associated with subjective, physiological, and neuroendocrine tolerance (Lee and Rivier, 1997, Lee et al., 2004, Richardson et al., 2008, King et al., 2006, King et al., 2011) and increased attentional bias to alcohol cues (Field et al., 2004, Cofresí et al., 2019, Barker et al., 2015), suggesting that higher binge levels of alcohol consumption lowers biobehavioral responses to acute alcohol, while at the same time increasing alcohol salience and increasing motivation for alcohol. However, the neural responses that may drive such increased alcohol motivation in the face of lower alcohol response has not been studied.
Previous neuroimaging research assessing effects of fixed doses of acute alcohol intake has reported lower brain response in reward regions such as the nucleus accumbens in alcohol users who binge relative to non-binge moderate users, consistent with the previously cited blunted subjective, physiological and neuroendocrine responses to alcohol found in binge users (Glimcher, 2011, Gilman et al., 2012, Kareken et al., 2004, Kareken et al., 2010). However, fixed dose acute alcohol effects on brain responses represent only the psychopharmacologic effect of alcohol at specified investigator-selected doses, and the brain regions and networks that may play a role in increased self-motivation for alcohol remain unclear. We reasoned that to understand neural responses associated with increased motivation after an initial drink it is important to quantify initial self-motivation for alcohol in a controlled manner, i.e., how much alcohol does a person want and consume relative to placebo alcohol, and at low levels equivalent to 1-2 drinks so as to benchmark initial motivation against future sustained motivation. Thus, we defined sustained motivation as an increase in motivation for alcohol relative to the initial alcohol self-motivation, and assessed both initial and sustained alcohol self-motivation in a controlled alcohol vs. placebo randomized experiment.
We previously utilized the Alcohol Taste Test (ATT), a well-known and valid test of ad-lib alcohol intake and also implicit alcohol motivation (Jones et al., 2016), in which individuals are asked to drink alcohol from two 12-oz beers and determine if the beers are same or different (Blaine et al., 2019). We found highly stable measurements of initial alcohol self-motivation of intake at low levels of 1-2 drinks across 3 experimental sessions (Cronbach α=0.85), and also evidence of alcohol-related neuroendocrine tolerance and high subjective craving in binge (BD) versus non-binge, social drinkers (SD) (Blaine et al., 2019). Thus, utilizing alcoholic and non-alcoholic beer for the alcohol and placebo sessions respectively, we used this previously validated ATT procedure to establish ‘initial’ alcohol self-motivation measured as the amount consumed in the alcohol and placebo sessions, akin to what may occur in the early phase of a drinking episode. Given that alcoholic and modern non-alcoholic (placebo) beer are highly similar in taste(Pieczonka et al., 2021, Melton, 2022) and the low amounts of beer availability in the pre-scan ATT, we expected no differences in initial self-motivation amounts in the pre-scan alcohol vs placebo session or by the BD and MD groups. However, given the findings cited above that binge drinkers have altered subjective, physiologic, endocrine and neural responses to specific doses of alcohol and also higher subjective craving in response to initial self- motivated consumption (Blaine et al., 2019), we hypothesized that there will be an increased sustained self-motivation in BD, relative to the SD, in the alcohol but not the placebo session, indicative of increased ‘sustained’ self-motivation for alcohol, i.e. seeking out more alcohol, relative to initial alcohol self-motivation.
To understand the neural responses that may drive the hypothesized sustained alcohol self-motivation after initial self-motivation during the early phase of a drinking episode, we adapted the ATT procedures to assess neural activity associated with initial self-motivation immediately prior to an functional magnetic resonance imaging (fMRI) scan in the pre-scan ATT and assessed sustained alcohol motivation immediately following the fMRI in a post-scan ATT, with each ATT assessing different aspects of self-motivation (i.e., initial and sustained) and with different predictions for each as outlined above. We utilized arterial spin labeling (ASL) perfusion fMRI that assesses cerebral blood flow (CBF) to assess neural responses to initial self-motivated consumption of alcoholic vs. non-alcoholic beer on two separate days in a randomized, placebo-controlled, single-blind cross-over design. ASL approaches have benefit over BOLD fMRI in that they provide a direct, absolute quantification of regional cerebral blood flow effects of acute pharmacologic drugs such as alcohol over resting/placebo cerebral blood flow, while BOLD fMRI only provides an indirect relative measure of neural activity and is susceptible to neurovascular coupling effects on cerebral blood flow and volume as discussed in previous research(Rickenbacher et al., 2011, Stewart et al., 2014, Chen et al., 2011, Geil et al., 2014). Based on previous research on neural effects of alcohol in BD and AUD samples, we hypothesized that BD would show blunted neural reward responses in the VS and also in the orbitofrontal cortex (OFC). Moreover, given the OFC’s role in alcohol reinforcement and flexible control of alcohol intake (Moorman, 2018, Balleine et al., 2011), we further hypothesized that the alcohol session- specific OFC blunting would be predictive of greater sustained behavioral alcohol self-motivation in the post-scan ATT in BD relative to SD.
Materials and Methods
Participants:
Beer drinking, non-smoking men and women ages 21-45 (N=52) with no physical and mental health disorders were recruited from the Greater New Haven area using online and social media advertisements during the period from September 2016 through June 2018 (see Supplemental Figure 1 for CONSORT Diagram). Prior to experimental sessions, the 90 day Timeline FollowBack (Sobell and Sobell, 1992) and Cahalan Quantity and Frequency Variability Index (Cahalan et al., 1969) interviews were used to assess recent drinking behavior. On the basis of the reported current alcohol intake patterns on these interviews, participants were classified into BD (N=27) and SD (N=25) groups utilizing established NIAAA binge drinking criteria (NIAAA, 2016). Using these criteria, the non-binging Social Drinkers (SD) were those who did not exceed 7 standard drinks/wk for women and 14 standard drinks/wk for males, with no occasions of binge drinking, and those who reported regular alcohol use in binges of 4 or more drinks per episode for women or 5 for men and at least 8 standard drinks/wk for women and at least 15 standard drinks/wk for men were classified as binge drinkers (BD). Current DSM-V psychiatric disorders, as assessed by the Structured Clinical Interview for DSM-5 (SCID-5)(First et al., 2015), including AUD, were exclusionary as were any prescription medications. Sample size estimates were based on effect size and power calculations from our previous experimental work utilizing the novel pre-scan ATT procedures to assess alcohol self-motivation (Blaine et al., 2019)) and from previous neuroimaging studies of acute fixed dose alcohol administration in BD and SD (Gilman et al., 2012). Groups were matched demographically (age, sex, race, education), on family history of AUD, years of regular drinking, current stress levels, and number of lifetime traumatic events (see Supplemental Table 1 for Participant Characteristics). All participants provided written informed consent and the study protocol was approved by the Yale University Human Investigation Committee. The study was registered on Clinicaltrials.gov (NCT03165942).
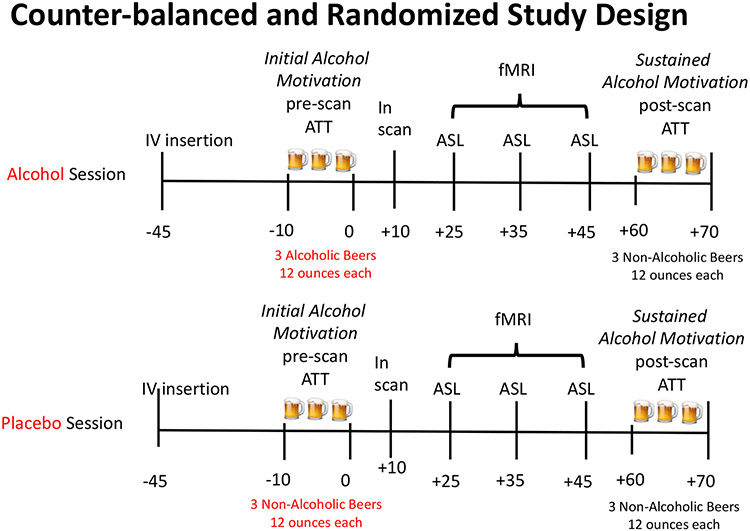
Experimental Design and Procedures (Figure1):
Figure 1. Experimental design.
Participants underwent two neurobehavioral experimental sessions initiated at 1:00 pm on each day, one week apart. During each session, an intravenous line for repeated measurement of blood cortisol levels was inserted 45 minutes prior to the session (−45 timepoint), followed by a pre-scan ATT assessment of initial alcohol self-motivation conducted with alcoholic beer (Alcohol) or non-alcoholic (Placebo) beer for 10 minutes. This was immediately followed by three, 5-minute blocks of Arterial Spin Label (ASL) perfusion MRI scanning procedure, for a total of 15 minutes ASL measurement and total time in the scanner of 55 minutes. Immediately post MRI scans, participants underwent a second, post-scan ATT with non-alcoholic beer in both sessions to assess sustained alcohol motivation. Measurements of breath alcohol level (BrAC), cortisol, subjective craving, stimulation and sedation were obtained before and after the pre-Scan ATT (−10, 0), during scanning (+10, +25, +35, +45) and at post-Scan ATT(+60, +70) timepoints.
Randomization and Blinding:
This study involved two fMRI scans, one week apart, using a single-blind, counter-balanced, randomized, placebo-controlled, cross-over design. Initial beer self-motivation Session (alcoholic vs. non-alcoholic) was a Within Subjects factor where each subject was exposed to alcohol and placebo alcoholic beer on separate scan days, while the binge and non-binge drinking grouping was a Between Subjects factor in the experimental design. Randomization was conducted by one of the investigators (SB) using an online random number generator program that assigned participants to the order of the placebo or alcohol session. The participant, MRI technologist and MRI research assistant were blind to the pre-scan ATT alcoholic and non-alcoholic beers used in each session.
Experimental Procedures:
Prior to each experimental scan session, participants were required to be drug free as tested by a urine drug screen for cannabis, benzodiazepines, opiates, and stimulants, and also alcohol-free using a Draeger breathalyzer. The 10-minute pre-Scan Alcohol Taste Test (pre-scan ATT) involved presenting the participant with 3 alcoholic (Alcohol) or non-alcoholic (Placebo) beers (total of 1065 ml) and instructing them to taste the beers to assess if they were the “same or different” kind of beer. They were also instructed to “drink as much as they need to” to make that determination and that they would be paid $10 if they are correct. Therefore, a participant could taste as much or as little as they wanted from each beer glass to make their decision. In the alcohol session, participants received beer with a 4.2% alcohol concentration during the pre-scan ATT. In the placebo session, participants were provided with non-alcoholic beer. Notably, participants often choose to consume more than a sip of each beer, and the amount consumed serves as a reliable behavioral index of alcohol self-motivation (Jones et al., 2016, Blaine et al., 2019). This placebo-controlled design with the opportunity to consume only low amounts of alcohol/placebo allowed for the isolation of the pharmacologic alcohol effect from expectancies and external cues, and thus the alcohol versus placebo contrast was utilized to assess initial alcohol self-motivation and alcohol drug effect in the alcohol versus the placebo session.
After the pre-Scan ATT on each test day, participants were placed in the 3T MRI and underwent three 5-minute runs of arterial spin labeling (ASL) perfusion imaging for a total of 15 minutes ASL measurement, which was accompanied by measurements of subjective stimulation, sedation, craving, cortisol, and breath alcohol levels. Arterial spin labeling of brain perfusion is an alternate measure to brain oxygenation level dependent (BOLD) measures, and is a direct and absolute measure of regional cerebral blood flow related to a specific stimulus. ASL is especially useful if that stimulus itself (i.e., alcohol) may have significant effects on the cerebrovascular environment and vascular flow (Rickenbacher et al., 2011, Stewart et al., 2014, Chen et al., 2011, Geil et al., 2014). On the other hand, BOLD fMRI relies on changes in the magnetic properties of oxygen-rich and oxygen-poor blood to map brain activity, while ASL uses a magnetic pulse to label arterial blood and calculate blood flow in the brain, providing a more direct quantitative measure. Total time in the scanner was 55 minutes.
During each scan session, breath alcohol levels, blood plasma samples for cortisol assessments, the Biphasic Alcohol Effects Questionnaire and Drug Effects Questionnaire (Martin et al., 1993, Morean et al., 2013), were administered at 8 timepoints (see Figure 1). Repeated plasma cortisol samples (4 ml each) were processed and analyzed using standard radioimmunoassay procedures. Breath alcohol levels were measured using the Draeger Alcotest 6820 (Lubeck, Germany) using a system developed by Gan et al. to obtain breath alcohol measurements in an MRI environment (Gan et al., 2014). Specifically, we obtained samples (approximately 1.5l) of end-expiratory air using a tube (PVC, 140 cm) connected to a standard children’s toy balloon (diameter of 75 cm), which was then quickly attached to the breathalyzer and the air blown through the device.
Assessment of Sustained Alcohol Self-Motivation:
Immediately following the MRI, participants underwent a post-scan ATT with only non-alcoholic beer in both alcohol and placebo beer sessions to assess sustained alcohol self-motivation as a function of the initial self-motivated amount consumed in the alcohol vs placebo assessment, and to isolate the specific neural effect of initial self-motivation of alcohol vs. placebo and its potential subsequent effect on sustained alcohol self-motivation versus the placebo session without introducing any further pharmacological alcohol effect.
Statistical Analyses:
Changes in breath alcohol levels, stimulation, sedation, craving, and cortisol responses over repeated time points were analyzed in the alcohol vs. placebo initial self-motivation sessions. The effect of Session was assessed using multilevel mixed effects models with Participants as the random factor, Group as a fixed factor, and timepoint and Session as repeated measures fixed factors. pre-scan ATT beer amount consumed was included as a co-variate in all subjective neuroendocrine and neuroimaging data analyses.
Neuroimaging Procedures and Analysis:
MRI scans were performed on a Siemens 3T Prisma scanner using a 64-channel head coil. A three-plane localizer was first acquired followed by a high-resolution whole-brain T1-weighted three-dimensional magnetization-prepared rapid gradient echo (MPRAGE) volume scan (Field-of-view (FOV): 256 x 256 mm2, Slice per slab: 208, Slice thickness: 1.0 mm, repetition time (TR): 1860 ms, echo time (TE): 1.96 ms, Flip Angle (FA): 8°, bandwidth: 240 Hz/Pixel, voxel size: 1.0x1.0x1.0 mm3). Cerebral blood flow (CBF) measured using the EPISTAR QUIPSS PASL with parameters as follows: FOV 220 x 220 mm2, matrix 88 x 88, bandwidth 2,185 Hz/pixel, slice thickness 3.6mm, TR 3000 ms, TE 20 ms, FA 90°. Data were converted from Digital Imaging and Communication in Medicine format to Analyze format using XMedCon. General linear models were used for individual-level analysis on each voxel in the entire brain volume. Trials with linear motion in excess of 1.5 mm or rotation greater than 2 mm were discarded. The number of trials discarded was not different between the BD and SD groups. Temporal filtering was carried out by including drift correction in the general linear model. Each trial was spatially smoothed using a 6-mm Gaussian kernel and individually normalized to generate beta maps (3.44 mm3).
To account for individual anatomical differences, three sequential registrations were performed using BioImage Suite: linear registration of raw data into two-dimensional anatomical images, the two-dimensional to three-dimensional (1.3 x 1.3 x 1 mm) linear registration, and a nonlinear registration to a reference three-dimensional image, the Colin27 Brain. Images were corrected for small participant motions (using SPM12) and high-pass filtered to remove baseline drift. After correction of the linear global drift on a per-voxel basis and in-plane low-pass filtering with a Gaussian kernel of 6 mm, time series of the perfusion-weighted images were obtained by pair-wise “surround” subtraction between interleaved label and control pairs, resulting in a temporal resolution of 2TR. The mean difference map was calculated by averaging all the difference images in the time series for each of the 3 ASL runs. The same low-pass filtering was applied to the proton-density image . Given and maps, the absolute CBF (ml/100g/minute) map was calculated using the Bloch Equation incorporating cerebral tissue perfusion terms, assuming , , , , , and the post-labeling delay time of each slice (Aguirre et al., 2002, Wong et al., 1997, Wang et al., 2003, Luh et al., 1999).
For group level data analysis, linear effects models using AFNI 3dLME (http://afni.nimh.nih.gov) was implemented with a 2 (session: Alcohol, Placebo) x 3 (run: 1, 2, 3) x 2 (group: BD, SD) design while covarying for pre-Scan ATT amount of beer consumed in milliliters, age, and sex. Session and run were treated as within-participant fixed-effect factors, group as a between participants factor, and participant as a random factor. To correct for multiple comparisons, we used family-wise errors (FWE) correction determined by Monte Carlo simulation using the AFNI 3dClustSim version (16.3.05, October 2016) program. Results are shown at p<0.05 cluster corrected with an initial whole brain analysis threshold of p<0.001 (9 voxels = 243 mm3). Despite our a priori circuit level hypotheses, we performed full brain analyses to increase rigor, as ROI analyses are susceptible to false positives (Eklund et al., 2016). Four participants completed only one scan (3 placebo, 1 active). Blood samples were not collected from 2 participants. Associations between changes in perfusion and subjective and behavioral measures were tested using two-way ANOVAs and/or linear regression.
Study approval:
The protocol was approved by the Yale University School of Medicine Human Investigation Committee. All participants provided informed, written consent before participation.
Results
Initial Alcohol Self-Motivation with the Pre-scan ATT:
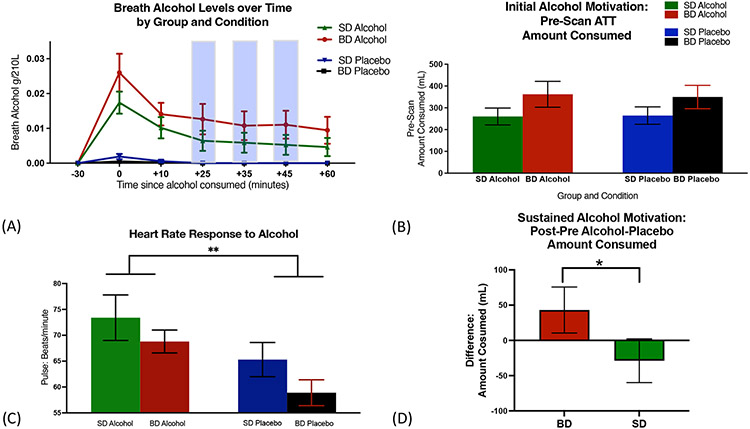
Across groups and sessions, participants were unable to distinguish the alcoholic vs placebo beer, χ2= 0.1, p= 0.99 and correctly guessed that the beers within each ATT were the same only at chance level, rendering the alcohol and placebo beers indistinguishable from each other. Findings show indistinguishable initial alcohol self-motivation in P\pre-scan ATT for alcohol and placebo sessions and similar breath alcohol levels (BrAC) in BD and SD, indicating equivalent initial alcohol self-motivation in the placebo and alcohol sessions and also across SD and BD groups (Fig 2A-2B). Furthermore, as expected, alcohol significantly increased heart rate relative to the placebo pre-Scan ATT session (F(1,100)=8.1, p=0.005; Fig 2C), documenting the well-known alcohol-related physiological stimulation effect, and with no differences between the BD and SD groups.
Figure 2. Alcohol versus placebo intake experimental manipulation:
(A) In the pre-scan ATT, groups did not differ in alcohol/placebo intake amounts (F(1,100)=0.03, p=0.87), nor within groups across sessions, (BD: t(52)=0.15, p=0.88; SD: t(50)=0.29, p=0.77), indicating no differences between groups in initial alcohol self-motivation. (B) Significantly higher breath alcohol levels were observed in the alcohol vs placebo Session (F(1,536)=121.5, p<0.0001) across both groups, with no group differences at any point during the experiment (Peak BrAC mean: BD - 0.026 +/− 0.005; SD - 0.017 +/− 0.003). (C) Heart rate assessed during initial 30 minutes after Pre-Scan ATT increased in both groups in the alcohol compared to placebo Session (F(1,100)=8.1, p=0.005). (D) Sustained alcohol self motivation assessed as change in the post-pre scan ATT for alcohol relative to the placebo session indicated BD consumed significantly more amounts than SD (t(50)=2.55, p=0.01, and with no differences between groups in the post- pre amount for the placebo session (t(50)=08, p=0.43).
Sustained Alcohol Self-Motivation with the Post-scan ATT:
Sustained alcohol self-motivation measured as change from initial self-motivation within person and in alcohol relative to placebo beer session revealed that BD showed significantly greater sustained alcohol self-motivation than SD (Fig 2D) only in the alcohol session and not in the placebo session.
Validation of the Alcohol Self=Motivation Responses:
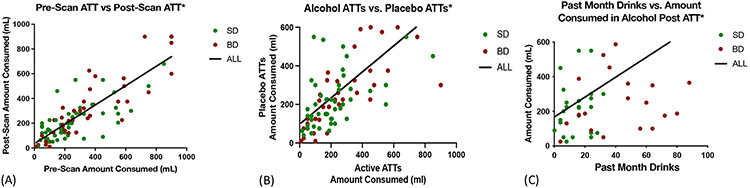
Notably and as expected, pre-Scan ATT and post-Scan ATT beer self-motivation amounts were found to be highly stable and consistent (Cronbach: ALL α=0.94, BD α=0.96, SD α=0.9, Fig 3A,B) and also significantly associated with real world alcohol intake levels, thereby providing ecological validity of the behavioral assessment of alcohol self-motivation (Fig 3C).
Figure 3. Reliability and Consistency of the ATT Procedures.
(A) The strongest predictor of the amount of alcohol consumed in the post-scan ATT was the amount consumed in the pre-scan ATT, [All: F(1,94)=238.4, p<0.0001, R2 =0.72; BD F(1,26)= 121.5, p<0.0001, R2 =0.62;SD F(1,24)=28.51, p<0.0001, R2 =0.53, so pre-scan ATT was included as a co-variate in all analyses. (B) Participant consumption of alcoholic and placebo beer was highly correlated, providing further evidence that participants were unable to distinguish the two,[All: F(1,92)= 95.1, p<0.0001, R2 =0.51, BD: F(1,25)=62.26, p<0.001, R2=0.74; SD: F(1,22)=38.72, p<0.0001, R2 =0.64.]. (C) The amount of alcohol consumed in the ATTs was strongly related to the participants’ regular self-reported monthly alcohol consumption for all participants, [All: F(1,50)= 10.84, p=0.002, R2 =0.22; BD: F(1,25)= 5.557, p=0.03, R2 =0.18; SD: F(1,23)=4.348, p=0.048, R2 =0.6.]
Subjective Ratings of Stimulation, Sedation, and Craving:
Lower stimulation in alcohol relative to placebo session was seen in the BD, while SD showed the opposite (Group X Session: F(1,531)=4.25, P=0.04). BD also showed lower sedation in the Alcohol relative to Placebo session, while SD again showed the opposite (Group X Session: F(1,557)=6.57, p=0.01). Additionally, BD experienced significantly greater craving in the Alcohol versus the Placebo Session whereas SD showed the opposite (Group X Session X Time: F(1,532)=6.09, p=0.01, Fig SF2A-F).
Plasma Cortisol Responses:
Consistent with previous research (Blaine et al., 2019), BD showed higher basal cortisol levels than SD (p<0.003, Fig SF3A). In response to the alcohol pre-scan ATT relative to placebo, the BD group showed neuroendocrine tolerance, whereas the SD group showed an increased cortisol response, (F(1,529)=4.62,p=0.03, Fig SF3B). This blunted cortisol response to pre-Scan ATT, in Alcohol versus Placebo Session, was negatively related to the sustained alcohol self-motivation amount consumed in the Alcohol post-Scan ATT in BD only, F(1,25)=7.68, p=0.01 (Fig SF3C).
Neural CBF Response During Alcohol relative to Placebo Scan:
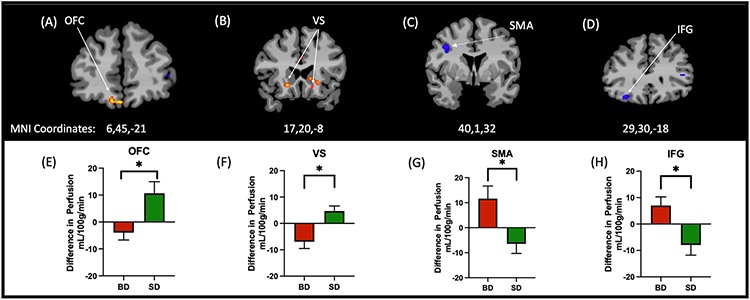
A significant Group x Session interaction was seen after whole brain correction at p<0.001 and cluster correction at 0.05. Marked blunted neural response was observed in brain reward regions of the OFC and VS for BD relative to SD (Fig 4A-B, E-F) as well as additional lower activation in the caudate, medial thalamus, dorsal anterior cingulate (data not shown, see Supplemental Table ST2). In contrast, BD showed greater activation in the Right inferior frontal gyrus (IFG) and supplementary motor area (SMA) vs. SD in the Alcohol vs Placebo sessions (Fig 4C-D,G-H).
Figure 4.
Whole brain CBF analyses of neural responses to alcohol vs placebo in SD vs BD groups, at p<0.001 threshold and cluster corrected p<0.05, with MNI coordinates x,y,z,. 4A-B, 3E-F: Evidence of neural reward tolerance was observed in the OFC and VS for the BD relative to SD. 4C-D, 4G-H: Inversely, BD showed greater activation than SD in the Right inferior frontal gyrus (IFG) and supplementary motor area (SMA). Note: Red/yellow denotes SD>BD, blue denotes BD>SD. Images are presented in radiological convention with the right side of the brain in the left side of the image.
Neural CBF Response associated with Differential Sustained Alcohol Self-Motivation:
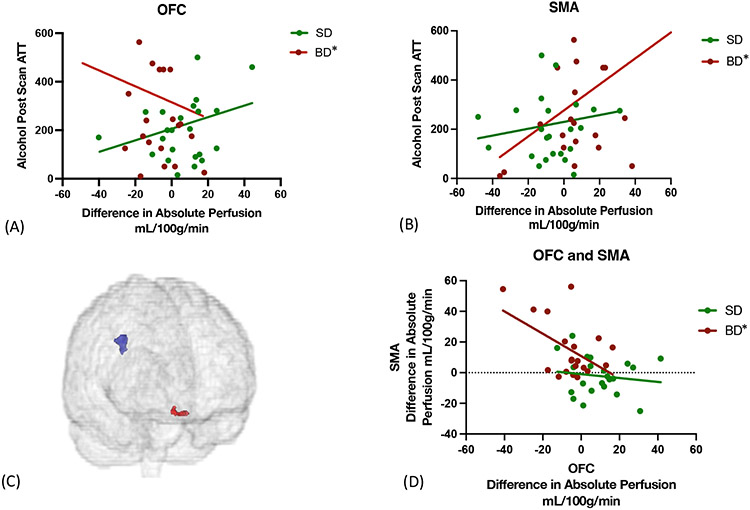
Notably, alcohol-related blunted OFC and a sensitized SMA responses each correlated with greater sustained alcohol self-motivation amount in the alcohol post-scan ATT session in BD, but not SD, with no significant relationship in the placebo session (Fig 5).
Figure 5. Relationship between alcohol induced CBF and Alcohol Session Post-Scan ATT in the BD Group.
A-B: Lower alcohol-induced CBF in the OFC (F(1,22)=6.5, p=0.02, , R2 =0.12) and SMA F(1,21)=6.5, p=0.02,R2 =0.24, of BD predicted higher subsequent post-scan ATT intake only in the alcohol session, indicative of both OFC and SMA playing a role in subsequent sustained alcohol-related motivation. C-D: Furthermore, lower alcohol-induced OFC response (red) correlated with concurrent sensitized SMA response (blue) in BD, F(1,18)=6.1,p=0.02. R2=0.25, but not in SD.
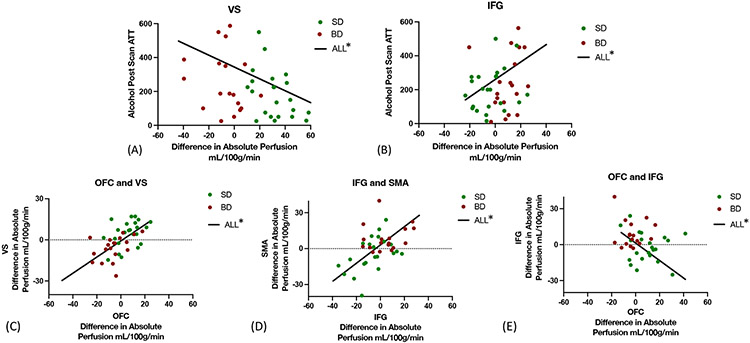
In contrast, lower VS (F(1,45)= 6.19, p=0.02) and higher right IFG CBF (F(1,45)= 5.01, p=0.03) were associated with higher sustained alcohol motivation amounts in the in the alcohol relative to placebo session for all participants. (Fig 6). Moreover, as expected, alcohol related CBF in the OFC was strongly positive associated with the VS CBF across all participants. Finally, lower OFC perfusion was also correlated with enhanced IFG perfusion across all participants (Fig 6).
Figure 6. Association between CBF responses in reward and behavioral intention regions and with Alcohol Session Post-Scan ATT in the Full Samples.
(A) The-Alcohol- Placebo difference in VS perfusion was significantly negatively related to post-scan ATT intake in the alcohol session for the full sample, but not in the separate groups, F(1,45)= 6.19, p=0.02, R2 =0.12. (B) The Alcohol- Placebo difference in IFG perfusion was significantly positively related to post-scan ATT intake in the alcohol session for the full sample, but not in the separate groups, F(1,45)=5.01, p=0.03, , R2 =0.1. (C) Alcohol- Placebo VS perfusion was positively associated with perfusion in the OFC, F(1,50) =36,94, p<0.0001, R2=0.48, in the full sample. (D) Alcohol-Placebo perfusion in the IFG and SMA were positively associated, F(1,50)=8.65,p=0.005, R2=0.45 in the full sample € Alcohol- Placebo OFC perfusion was significantly negatively related to IFG perfusion, F(1,50)= 11.12, p=0.004, R2=0.17 in the full sample.
Discussion
Current findings identified potential neurobehavioral processes that may drive sustained alcohol motivation in a single drinking bout in at-risk binge versus non-binge alcohol users. Using a placebo-controlled, randomized, cross-over clinical experiment, we showed that the neural response to initial self-motivated intake of low amounts of alcoholic but not placebo beer was associated with a sustained increase in alcohol self-motivation in BD and not SD. The validity of the experimental procedure was demonstrated in three ways. First, participant behavior in this protocol related significantly to real-world drinking behavior. Second, participants were unable to tell the difference between active alcoholic and non-alcoholic placebo beer as evidenced by equivalent amounts of pre-scan ATT amounts consumed across both sessions. Finally, the experimental manipulation to benchmark initial alcohol self-motivation was successful in that there were no differences between BD vs SD in beer amounts consumed across alcohol and placebo sessions and across groups. Therefore, the subjective, neuroendocrine, and neural differences seen between groups were not related to conscious alcohol expectations and all effects were seen while controlling for individuals’ pre-scan ATT consumption. As there were no differences in initial alcohol-self motivation between groups or across alcohol vs. placebo sessions, the increases in sustained alcohol self-motivation may be attributable to the differences between groups in neural, subjective and endocrine response to acute initial alcohol self-motivation, above and beyond any differences between groups in drinking history.
Importantly, we demonstrated alcohol-specific blunted neural reward responses in the BD relative to SD group, that in turn contributed to sustained increases in alcohol self-motivation. Specifically, SD showed expected alcohol-induced increased CBF in VS and OFC regions in the alcohol vs placebo contrast. The VS and OFC regions are associated with alcohol reward (Boileau et al., 2003, Stalnaker et al., 2018) and have also been associated with predicting receipt of drug reward (Schultz et al., 1993), but the BD group showing alcohol-induced blunted neural reward responses is suggestive of neural reward tolerance. Tolerance is an automatic neurophysiologic learned signal (Siegel, 1977), observed here in key regions associated with drug reinforcement, and possibly signaling lower reward, only in the alcohol and not in the placebo session, and only in the BD group. Furthermore, the alcohol-induced OFC tolerance (i.e., blunted activation) in BD was significantly associated with sustained increases in alcohol self-motivation in the alcohol, but not placebo session. Notably, these results are consistent with previous work showing neural opioid tolerance increasing heroin self-administration and drug seeking in laboratory animals (Xi et al., 2004, Luo et al., 2004), and in previous human studies where OFC-striatal hypoactivation predicted escalated alcohol intake in individuals with AUDs (Blaine et al., 2020, Volkow et al., 2017, Martinez and Narendran, 2010). While a history of binge drinking clearly distinguishes the group in their neural, subjective ad endocrine responses to initial alcohol-self motivation in the alcoholic vs. placebo beer sessions, the placebo-controlled experimental nature of this study permitted assessment of acute sustained increase in self-motivation within a single session above and beyond overall group differences due to drinking history. The findings across the neural, subjective, endocrine and sustained alcohol self-motivation responses show differences between alcohol vs placebo responses which points to specific alcohol pharmacologic effects at the low alcohol intake levels.
The blunted response of the OFC to alcohol-relative to placebo and its association with increased sustained alcohol motivation only in BD suggests that neural tolerance in reward regions contributes to sustained alcohol-self-motivation. The OFC has been described as necessary for flexible, motivated behavior (Moorman, 2018), in addition to reward evaluation of sensory input that underlies alcohol intake behavior (Rolls, 2015). Notably, blunted OFC in BD was correlated with concurrent sensitized SMA blood flow, suggesting that the tolerant OFC signal may be related to sensitized neurobehavioral motivational intention signal in the SMA(Lee et al., 2017, Bari and Robbins, 2013, Aron et al., 2014). Thus, we speculate that the blunted OFC response may represent a right shift in the learned neurophysiologic positive reinforcement set-point signal, that in turn, may promote sustained neurobehavioral alcohol motivation in BD. These findings suggest a significant role of neural reward tolerance, independent of negative reinforcement mechanisms in escalating alcohol intake prior to development of AUD and consistent with other pre-clinical and clinical studies. For example, in rats,, inactivation of the medial PFC produced the same effects as training doses of alcohol on the medial PFC’s cortical and thalamic connections, suggesting a clear role for deactivation of the PFC in response to alcohol in a model of AUD (Jaramillo et al., 2016). Moreover, the OFC is also highly interconnected with the other regions in which SD show greater alcohol related activation, i.e., the striatum. Chronic alcohol consumption alters these prefrontal-striatal connections and such changes are linked to steeper delay discounting in drinkers (Arias et al., 2021).
On the other hand, BD showed greater perfusion in areas associated with behavioral intention, motor planning and attentional control, i.e., right IFG and right SMA. This greater CBF in the SMA in BD was positively and significantly related to greater sustained alcohol self-motivation. Heightened excitability in the prefrontal motor cortex has been seen in people with AUD post detoxication (Naim-Feil et al., 2014) and has been linked to compensatory upregulation of NMDA glutamate receptors (Gass and Olive, 2008, Tzschentke and Schmidt, 2003). Preclinical studies utilizing low alcohol concentrations, modeling comparable amounts of alcohol, have shown that acute exposure to alcohol increases the intrinsic excitability of neurons, especially in animals with previous chronic alcohol exposure (Signore and Yeh, 2000). Moreover, disrupted motor timing has been linked to treatment outcomes and alcohol self-efficacy (Young et al., 2018) and may be related to these changes in intrinsic excitability of motor cortices.
Behavioral intention to continue consuming alcohol has repeatedly been shown to predict future drinking behavior (Cooke et al., 2016), but the neural underpinnings of such intentions have not been well understood thus far. Previous work with non-binge social drinkers found that an extremely small, fixed dose of alcohol (10 ml) resulted in greater IFG response to the taste of alcoholic than to non-alcoholic beer, but decreased activation in the SMA (Smeets and de Graaf, 2019). Our study is unique in that the dose was not fixed for participants and was driven by participants’ initial alcohol-self-motivation. Thus, we suggest this pattern of activation may be associated with greater sustained alcohol self-motivation as shown in the active but not the placebo session post-scan ATT.
Other previous studies have examined the effect of alcohol consumption on perfusion, but with key differences in study design, and critically, not examining the effects on alcohol self-motivation. These differences include the time of perfusion measurement related to alcohol consumption and the use of a fixed dose of alcohol, thus removing any self-driven alcohol motivation component. Across these studies, alcohol consumption was generally associated with increased CBF in the brain overall and specifically in the frontal cortex(Khalili-Mahani et al., 2011, Rickenbacher et al., 2011, Marxen et al., 2014). However, only one study used a larger sample than the current study (Tolentino et al., 2011), while all others used an N smaller than either of our groups. That study reported results similar to current findings, specifically, that individuals who show low response to alcohol (a genetically influenced risk factor associated with greater motivation for alcohol), showed lower CBF throughout the frontal cortex than those who are more responsive, despite equivalent blood alcohol levels, 0.06mg%. Moreover, a smaller study that utilized intravenous alcohol infusions also showed lower CBF response in the dorsal anterior cingulate of low responders at 0.08mg% (Strang et al., 2015). Despite the clear differences in design and purpose between the current study and this previous work, the current findings generally align with previous studies.
It is important to note that despite the powerful cross-over within-person and between group experimental design, this study is limited by the smaller numbers of women included in the sample which prevented examination of sex-related differences in neuroendocrine and neural response to alcohol. Also, within the BD group, none of the participants met criteria for moderate-severe AUDs at the time of the study and it is unknown which, if any, would go on to develop moderate-severe AUDs. Additionally, although we carefully obtained drinking histories on separate days and across measures prior to SD and BD classification, no biochemical verification of current alcohol use was performed. Finally, despite high correlation with real world behavior, these findings are limited by the laboratory context of the experiment.
These limitations notwithstanding, we present novel findings utilizing a unique experimental paradigm to understand the neural correlates of sustained increases in alcohol self-motivation which was benchmarked to initial alcohol self-motivation with-in a single session in groups who were well defined in terms of their current binge vs non-binge alcohol drinking patterns. We found that, compared to SD and after controlling for placebo effects, BD have less reward activation in response to initial self- motivated consumption of low levels of alcohol in the OFC, which in turn was associated with greater sustained alcohol self-motivation in the post-Scan ATT. BD also show greater neural activation in the SMA related to increased behavioral intention to consume alcohol that was associated with both the blunted alcohol-related OFC activation and also significantly related to greater alcohol self-motivation in the post-Scan ATT. These findings suggest that both acute alcohol-related neural reward tolerance in the OFC and pre-motor sensitization response in the SMA may underlie escalated alcohol motivation and contribute to excessive alcohol intake even prior to development of alcohol use disorders. Findings imply that resetting of positive reinforcement regulation in the OFC and neural reward circuits could be of benefit in reducing risk of escalated alcohol motivation in alcohol users who binge drink.
Supplementary Material
Acknowledgments
Funding:
National Institutes of Health grant R21-AA025277 (RS), R01-AA013892 (RS), National Institutes of Health grant R00-AA02540 (SKB).
Footnotes
Competing Interest Statement: The authors have no competing interests to disclose.
References
- AGUIRRE GK, DETRE JA, ZARAHN E & ALSOP DC 2002. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage, 15, 488–500. [DOI] [PubMed] [Google Scholar]
- ARIAS AJ, MA L, BJORK JM, HAMMOND CJ, ZHOU Y, SNYDER A & MOELLER FG 2021. Altered effective connectivity of the reward network during an incentive-processing task in adults with alcohol use disorder. Alcoholism: Clinical and Experimental Research, 45, 1563–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARON AR, ROBBINS TW & POLDRACK RA 2014. Inhibition and the right inferior frontal cortex: one decade on. Trends in cognitive sciences, 18, 177–185. [DOI] [PubMed] [Google Scholar]
- BALLEINE BW, LEUNG BK & OSTLUND SB 2011. The orbitofrontal cortex, predicted value, and choice. Annals of the New York Academy of Sciences, 1239, 43–50. [DOI] [PubMed] [Google Scholar]
- BARI A & ROBBINS TW 2013. Inhibition and impulsivity: behavioral and neural basis of response control. Progress in neurobiology, 108, 44–79. [DOI] [PubMed] [Google Scholar]
- BARKER JM, CORBIT LH, ROBINSON DL, GREMEL CM, GONZALES RA & CHANDLER LJ 2015. Corticostriatal circuitry and habitual ethanol seeking. Alcohol 49(8), 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAINE SK, MILIVOJEVIC V, FOX H & SINHA R 2016. Alcohol effects on stress pathways: impact on craving and relapse risk. The Canadian Journal of Psychiatry, 61, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAINE SK, NAUTIYAL N, HART R, GUARNACCIA J & SINHA R 2019. Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non-binge drinkers. Addiction biology, 24, 1096–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAINE SK, WEMM S, FOGELMAN N, LACADIE C, SEO D, SCHEINOST D & SINHA R 2020. Association of prefrontal-striatal functional pathology with alcohol abstinence days at treatment initiation and heavy drinking after treatment initiation. American Journal of Psychiatry, 177, 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOILEAU I, ASSAAD JM, PIHL RO, BENKELFAT C, LEYTON M, DIKSIC M, TREMBLAY RE & DAGHER A 2003. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse, 49, 226–231. [DOI] [PubMed] [Google Scholar]
- BURKE CJ & TOBLER PN 2016. Time, not size, matters for striatal reward predictions to dopamine. Neuron, 91, 8–11. [DOI] [PubMed] [Google Scholar]
- CAHALAN D, CISIN IH & CROSSLEY HM 1969. American drinking practices: A national study of drinking behaviors and attitudes. Monographs of the Rutgers Center of Alcohol Studies, 6. [Google Scholar]
- CHEN Y, WANG Z & DETRE J Impact of equilibrium magnetization of blood on ASL quantification. Proceedings of the 19th Annual Meeting of ISMRM, Montréal, Canada, 2011. 300. [Google Scholar]
- COFRESÍ RU, BARTHOLOW BD & PIASECKI TM 2019. Evidence for incentive salience sensitization as a pathway to alcohol use disorder. Neuroscience & Biobehavioral Reviews, 107, 897–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOKE R, DAHDAH M, NORMAN P & FRENCH DP 2016. How well does the theory of planned behaviour predict alcohol consumption? A systematic review and meta-analysis. Health psychology review, 10, 148–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EKLUND A, NICHOLS TE & KNUTSSON H 2016. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the national academy of sciences, 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIELD M, MOGG K, ZETTELER J & BRADLEY BP 2004. Attentional biases for alcohol cues in heavy and light social drinkers: the roles of initial orienting and maintained attention. Psychopharmacology, 176, 88–93. [DOI] [PubMed] [Google Scholar]
- FIRST MB, WILLIAM JBW, KARG RS & SPITZER RL 2015. Structured Clinical Interview for DSM-V- Research Version (SCID 5 for DSM-5, Research Version; SCID-5-RV, Arlington, VA, American Psychiatric Association. [Google Scholar]
- GAN G, GUEVARA A, MARXEN M, NEUMANN M, JÜNGER E, KOBIELLA A, MENNIGEN E, PILHATSCH M, SCHWARZ D & ZIMMERMANN US 2014. Alcohol-induced impairment of inhibitory control is linked to attenuated brain responses in right fronto-temporal cortex. Biological psychiatry, 76, 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASS JT & OLIVE MF 2008. Glutamatergic substrates of drug addiction and alcoholism. Biochemical pharmacology, 75, 218–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEIL CR, HAYES DM, MCCLAIN JA, LIPUT DJ, MARSHALL SA, CHEN KY & NIXON K 2014. Alcohol and adult hippocampal neurogenesis: promiscuous drug, wanton effects. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 54, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILMAN JM, RAMCHANDANI VA, CROUSS T & HOMMER DW 2012. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology, 37, 467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLIMCHER PW 2011. Understanding dopamine and reinforcement learning: the dopamine reward prediction error hypothesis. Proceedings of the National Academy of Sciences, 108, 15647–15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARAMILLO AA, RANDALL PA, FRISBEE S & BESHEER J 2016. Modulation of sensitivity to alcohol by cortical and thalamic brain regions. European Journal of Neuroscience, 44, 2569–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES A, BUTTON E, ROSE AK, ROBINSON E, CHRISTIANSEN P, DI LEMMA L & FIELD M 2016. The ad-libitum alcohol ‘taste test’: secondary analyses of potential confounds and construct validity. Psychopharmacology, 233, 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAREKEN DA, BRAGULAT V, DZEMIDZIC M, COX C, TALAVAGE T, DAVIDSON D & O'CONNOR SJ 2010. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage, 50, 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAREKEN DA, CLAUS ED, SABRI M, DZEMIDZIC M, KOSOBUD AE, RADNOVICH AJ, HECTOR D, RAMCHANDANI VA, O'CONNOR SJ & LOWE M 2004. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: preliminary findings. Alcoholism: Clinical and Experimental Research, 28, 550–557. [DOI] [PubMed] [Google Scholar]
- KHALILI-MAHANI N, VAN OSCH MJ, BAERENDS E, SOETER RP, DE KAM M, ZOETHOUT RW, DAHAN A, VAN BUCHEM MA, VAN GERVEN JM & ROMBOUTS SA 2011. Pseudocontinuous arterial spin labeling reveals dissociable effects of morphine and alcohol on regional cerebral blood flow. Journal of Cerebral Blood Flow & Metabolism, 31, 1321–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING A, MUNISAMY G, DE WIT H & LIN S 2006. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol, 59, 203–9. [DOI] [PubMed] [Google Scholar]
- KING AC, DE WIT H, MCNAMARA PJ & CAO D 2011. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of general psychiatry, 68, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOX J, HASIN DS, LARSON FR & KRANZLER HR 2019. Prevention, screening, and treatment for heavy drinking and alcohol use disorder. The Lancet Psychiatry, 6, 1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE H-J, LIN F-H & KUO W-J 2017. The neural mechanism underpinning balance calibration between action inhibition and activation initiated by reward motivation. Scientific reports, 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE S & RIVIER C 1997. An initial, three-day-long treatment with alcohol induces a long-lasting phenomenon of selective tolerance in the activity of the rat hypothalamic–pituitary–adrenal axis. Journal of Neuroscience, 17, 8856–8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE S, SELVAGE D, HANSEN K & RIVIER C 2004. Site of action of acute alcohol administration in stimulating the rat hypothalamic-pituitary-adrenal axis: comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology, 145, 4470–4479. [DOI] [PubMed] [Google Scholar]
- LEYTON M, BOILEAU I, BENKELFAT C, DIKSIC M, BAKER G & DAGHER A 2002. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C] raclopride study in healthy men. Neuropsychopharmacology, 27, 1027–1035. [DOI] [PubMed] [Google Scholar]
- LUH WM, WONG EC, BANDETTINI PA & HYDE JS 1999. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 41, 1246–1254. [DOI] [PubMed] [Google Scholar]
- LUO F, XI Z-X, WU G, LIU C, GARDNER EL & LI S-J 2004. Attenuation of brain response to heroin correlates with the reinstatement of heroin-seeking in rats by fMRI. Neuroimage, 22, 1328–1335. [DOI] [PubMed] [Google Scholar]
- MARTIN CS, EARLEYWINE M, MUSTY RE, PERRINE MW & SWIFT RM 1993. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res, 17, 140–6. [DOI] [PubMed] [Google Scholar]
- MARTINEZ D & NARENDRAN R 2010. Imaging neurotransmitter release by drugs of abuse. Behavioral neuroscience of drug addiction, 219–245. [DOI] [PubMed] [Google Scholar]
- MARXEN M, GAN G, SCHWARZ D, MENNIGEN E, PILHATSCH M, ZIMMERMANN US, GUENTHER M & SMOLKA MN 2014. Acute effects of alcohol on brain perfusion monitored with arterial spin labeling magnetic resonance imaging in young adults. J Cereb Blood Flow Metab, 34, 472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELTON L 2022. Synbio salvages alcohol-free beer. Nature Biotechnology, 40, 8–8. [DOI] [PubMed] [Google Scholar]
- MOORMAN DE 2018. The role of the orbitofrontal cortex in alcohol use, abuse, and dependence. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 87, 85–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOREAN ME, DE WIT H, KING AC, SOFUOGLU M, RUEGER SY & O’MALLEY SS 2013. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology, 227, 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAIM-FEIL J, FITZGERALD PB, BRADSHAW JL, LUBMAN DI & SHEPPARD D 2014. Neurocognitive deficits, craving, and abstinence among alcohol-dependent individuals following detoxification. Archives of Clinical Neuropsychology, 29, 26–37. [DOI] [PubMed] [Google Scholar]
- NIAAA 2016. Drinking levels defined. [Google Scholar]
- PIECZONKA SA, PARAVICINI S, RYCHLIK M & SCHMITT-KOPPLIN P 2021. On the trail of the German Purity Law: Distinguishing the metabolic signatures of wheat, corn and rice in beer. Frontiers in Chemistry, 9, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON HN, LEE SY, O'DELL LE, KOOB GF & RIVIER CL 2008. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. The European journal of neuroscience, 28, 1641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICKENBACHER E, GREVE DN, AZMA S, PFEUFFER J & MARINKOVIC K 2011. Effects of alcohol intoxication and gender on cerebral perfusion: an arterial spin labeling study. Alcohol, 45, 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROLLS ET 2015. Taste, olfactory, and food reward value processing in the brain. Progress in Neurobiology 64–90. [DOI] [PubMed] [Google Scholar]
- SAMHSA 2020. Key substance use and mental health indicators in the United States: results from the 2019 National Survey on Drug Use and Health (Substance Abuse and Mental Health Services Administration; ) [Google Scholar]
- SCHULTZ W, APICELLA P & LJUNGBERG T 1993. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. Journal of neuroscience, 13, 900–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGEL S 1977. Morphine tolerance acquisition as an associative process. Journal of Experimental Psychology: Animal Behavior Processes, 3, 1. [DOI] [PubMed] [Google Scholar]
- SIGNORE AP & YEH HH 2000. Chronic Exposure to Ethanol Alters GABAAReceptor-Mediated Responses of Layer II Pyramidal Cells in Adult Rat Piriform Cortex. Journal of neurophysiology, 84, 247–254. [DOI] [PubMed] [Google Scholar]
- SMEETS PA & DE GRAAF C 2019. Brain Responses to Anticipation and Consumption of Beer with and without Alcohol. Chemical senses, 44, 51–60. [DOI] [PubMed] [Google Scholar]
- SOBELL LC & SOBELL MB 1992. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In: ALLEN L & LITTEN R (eds.) Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press. [Google Scholar]
- STALNAKER TA, LIU T-L, TAKAHASHI YK & SCHOENBAUM G 2018. Orbitofrontal neurons signal reward predictions, not reward prediction errors. Neurobiology of learning and memory, 153, 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART SB, KOLLER JM, CAMPBELL MC & BLACK KJ 2014. Arterial spin labeling versus BOLD in direct challenge and drug-task interaction pharmacological fMRI. PeerJ, 2, e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANG NM, CLAUS ED, RAMCHANDANI VA, GRAFF-GUERRERO A, BOILEAU I & HENDERSHOT CS 2015. Dose-dependent effects of intravenous alcohol administration on cerebral blood flow in young adults. Psychopharmacology, 232, 733–744. [DOI] [PubMed] [Google Scholar]
- TOLENTINO NJ, WIERENGA CE, HALL S, TAPERT SF, PAULUS MP, LIU TT, SMITH TL & SCHUCKIT MA 2011. Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcoholism: Clinical and Experimental Research, 35, 1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TZSCHENTKE T & SCHMIDT W 2003. Glutamatergic mechanisms in addiction. Molecular psychiatry, 8, 373–382. [DOI] [PubMed] [Google Scholar]
- VOLKOW ND, WIERS CE, SHOKRI-KOJORI E, TOMASI D, WANG G-J & BALER R 2017. Neurochemical and metabolic effects of acute and chronic alcohol in the human brain: studies with positron emission tomography. Neuropharmacology, 122, 175–188. [DOI] [PubMed] [Google Scholar]
- WANG J, AGUIRRE GK, KIMBERG DY, ROC AC, LI L & DETRE JA 2003. Arterial spin labeling perfusion fMRI with very low task frequency. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 49, 796–802. [DOI] [PubMed] [Google Scholar]
- WONG EC, BUXTON RB & FRANK LR 1997. Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In Vivo, 10, 237–249. [DOI] [PubMed] [Google Scholar]
- XI ZX, WU G, STEIN EA & LI SJ 2004. Opiate tolerance by heroin self-administration: An fMRI study in rat. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 52, 108–114. [DOI] [PubMed] [Google Scholar]
- YOUNG SY, KIDD M, VAN HOOF JJ & SEEDAT S 2018. Prognostic value of motor timing in treatment outcome in patients with alcohol-and/or cocaine use disorder in a rehabilitation program. Frontiers in psychology, 9, 1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.