Background:
Respiratory and urinary tract infections are frequent complications in patients with severe stroke. Stroke-associated infection is mainly due to opportunistic commensal bacteria of the microbiota that may translocate from the gut. We investigated the mechanisms underlying gut dysbiosis and poststroke infection.
Methods:
Using a model of transient cerebral ischemia in mice, we explored the relationship between immunometabolic dysregulation, gut barrier dysfunction, gut microbial alterations, and bacterial colonization of organs, and we explored the effect of several drug treatments.
Results:
Stroke-induced lymphocytopenia and widespread colonization of lung and other organs by opportunistic commensal bacteria. This effect correlated with reduced gut epithelial barrier resistance, and a proinflammatory sway in the gut illustrated by complement and nuclear factor-κB activation, reduced number of gut regulatory T cells, and a shift of gut lymphocytes to γδT cells and T helper 1/T helper 17 phenotypes. Stroke increased conjugated bile acids in the liver but decreased bile acids and short-chain fatty acids in the gut. Gut fermenting anaerobic bacteria decreased while opportunistic facultative anaerobes, notably Enterobacteriaceae, suffered an expansion. Anti-inflammatory treatment with a nuclear factor-κB inhibitor fully abrogated the Enterobacteriaceae overgrowth in the gut microbiota induced by stroke, whereas inhibitors of the neural or humoral arms of the stress response were ineffective at the doses used in this study. Conversely, the anti-inflammatory treatment did not prevent poststroke lung colonization by Enterobacteriaceae.
Conclusions:
Stroke perturbs homeostatic neuro-immuno-metabolic networks facilitating a bloom of opportunistic commensals in the gut microbiota. However, this bacterial expansion in the gut does not mediate poststroke infection.
Keywords: bacteria, bile acids, brain, infection, inflammation, ischemia
Infection is the most common complication after stroke affecting around 30% of the patients. Poststroke infection is associated with increased mortality and neurological deterioration.1 The most frequent infections are in the respiratory and urinary tracts, and the best clinical predictors of infection are the severity of the neurological deficit and the volume of the brain lesion. Prophylactic administration of antibiotics reduced overall infections but did not improve stroke functional outcome.2 Stroke-induced immunodepression is critically involved in poststroke infection. Reported mechanisms include a stress reaction mediated by the sympathetic nervous system and hypothalamic-pituitary-adrenal axis3–6 and inflammasome signaling in immune cells.7 The best-established elements of stroke-induced immunodepression are increased levels of stress hormones and anti-inflammatory cytokines, and lymphopenia.4
The microorganisms most often detected in patients with stroke-associated infection are opportunistic bacteria typical of the human intestinal tract. Translocation and dissemination of gut microbiota has been proposed as the cause of poststroke infection.8 Previous works reported imbalances in gut microbial populations after experimental and human stroke associated to worse outcome.9–15 Deranged microbiota composition, called dysbiosis, may have pathological consequences. Gastrointestinal metabolites, such as short-chain fatty acids (SCFAs)16 and bile acids (BAs),17 are important regulators of intestinal homeostasis affecting inflammatory, immune, and metabolic responses that can modify the microbial composition. The current view is that the gut microbiota influences stroke outcome and it is a putative therapeutic target in stroke.18,19
This study was aimed at identifying immunometabolic networks capable of dysregulating the composition of the gut microbiota after stroke, assessing drug treatments that may block this phenomenon, and investigating whether this effect may also reduce poststroke infection.
Methods
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Further details are provided in Supplemental Methods.
Animals
We used male wild-type C57BL/6 mice aged 11 to 12 weeks. Mice were from the same supplier (Janvier) except for one study designed to validate results in mice from a different supplier (Harlan-Envigo). In one experiment, we used reporter Cx3cr1eGFP/+ (CX3C motif chemokine receptor 1) male and female mice. Animal work was conducted according to the Spanish law (Real Decreto 53/2013) in compliance with the EU Directive 2010/63/EU for animal experiments and with approval of the local ethics committees. We report the study following the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Brain Ischemia
We occluded the middle cerebral artery for 45 minutes with an intraluminal filament.
Drug Treatments
Mice received either propranolol,3 N-phenyl-4-(3-phenylthioureido)benzenesulfonamide (LED209),20 RU486,3,6 or pyrrolidine dithiocarbamate (PDTC). For each mouse, stool samples were collected before surgery and treatments, and again 48 hours after reperfusion before euthanasia. Researchers blinded to the treatments processed the samples and performed the analyses.
In Vivo Bacterial Imaging
Mice received antibiotics and bioluminescent Escherichia coli21 was orally administered. At 24 hours, mice were subjected to stroke or sham surgery and were studied at 48 hours.
Metabolomics
BAs were extracted from liver and cecal contents for untargeted-based metabolomics using an HPLC system coupled to a mass spectrometer.22
Analysis of Fecal Microbial Groups by 16S rRNA Gene Profiling and Quantitative Polymerase Chain Reaction
Bacterial genomic DNA was isolated from feces, and the hypervariable V3 to V4 region of the 16S rRNA gene was amplified and processed on Illumina platform. Absolute bacterial levels were determined by quantitative polymerase chain reaction.23
Microbiological Analysis of Mouse Tissues
Bacteria in colony-forming units grown in agar plates seeded with tissue homogenates were identified by matrix-assisted laser desorption/ionization-time-offlight mass spectrometry mass spectrometry or partial sequence analysis of the 16S rRNA gene.
Statistics
The number of mice per group, statistical tests, and the P values are indicated in the figure legends. Statistical analyses were performed with GraphPad Prism version 8.3.0 or were computed on the software R v3.2.5 and MicrobiomeAnalyst.
Results
Poststroke Bacterial Infection
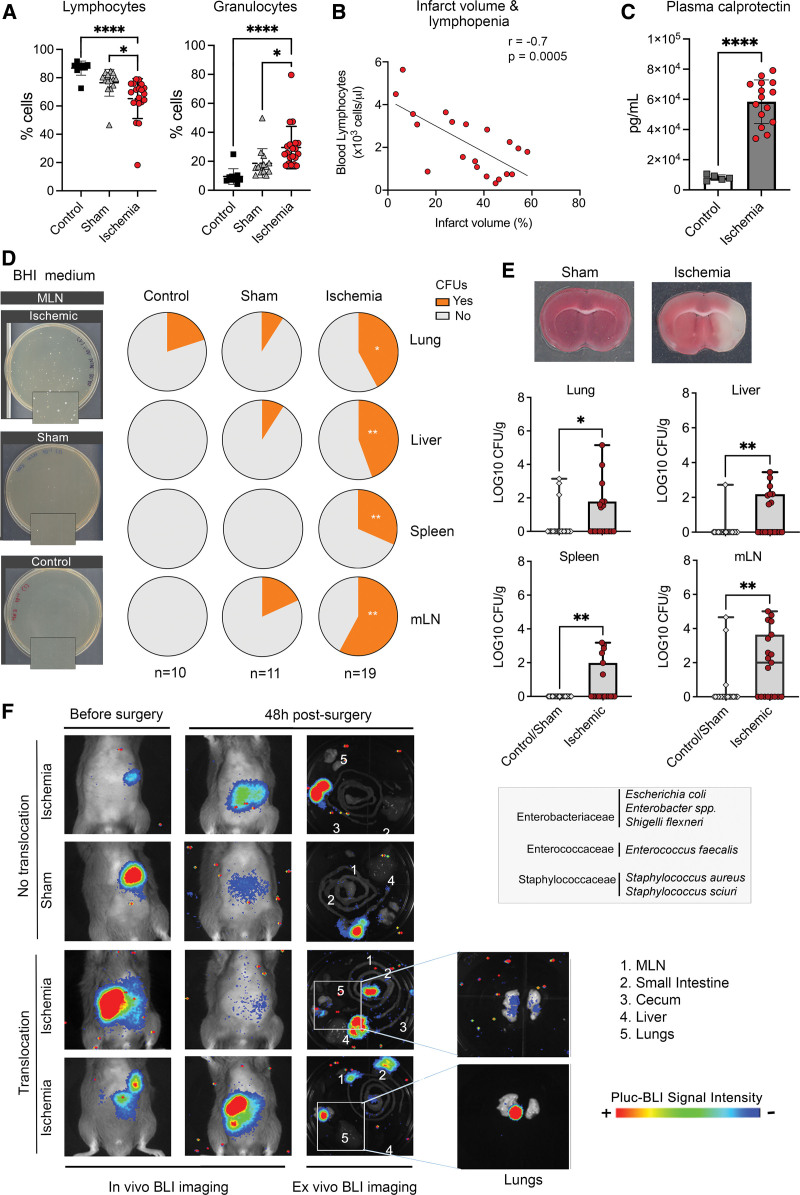
Our experimental ischemia model in mice induced marked blood lymphocytopenia and neutrophilia (Figure 1A) and reduced spleen and body weight (Figure S1A). Moreover, lymphocyte counts decreased as a function of stroke severity (Figure 1B), whereas this relation was not found for granulocytes (Figure S1B). Plasma calprotectin, a marker of infection/inflammation, increased 2 days postischemia (Figure 1C; Figure S1C). Aiming to assess poststroke infection, we determined colony-forming units (Figure 1D) in brain heart infusion agar plates after incubation of mesenteric lymph nodes (mLN), liver, spleen, or lung tissue homogenates obtained 2 days after ischemia or sham operation, and naïve controls. Sham-operated and control groups showed no differences (Figure 1D; Figure S1D) and were pooled together for analysis versus the ischemic group. The ischemic group showed higher incidence of microbial colonies (Figure 1D) and more microbial counts (Figure 1E). Isolated colonies identified by partial 16S rRNA gene sequencing showed the predominance of facultative anaerobic bacteria typical of gut microbiota represented by members of Gram-negative Enterobacteriaceae, including Enterobacter spp., E. coli, and Shigella flexneri, and of Gram-positive Enterococcaceae, that is, Enterococcus faecalis. We also detected Staphylococcus aureus and Staphylococcus sciuri.
Figure 1.
Ischemia-induced immune depression and bacterial growth. Mice were studied 2 d after ischemia or sham operation, and controls. A, Ischemia (n=19) reduced blood lymphocytes vs sham (n=14; *P=0.011) and controls (n=10; ****P<0.0001), and increased granulocytes vs sham (*P=0.021) and controls (****P<0.0001); 1-way ANOVA/Šídák test). B, After ischemia, blood lymphocyte number was inversely correlated with lesion volume (Pearson r=−0.7, P=0.0005, n=19). C, Ischemia (n=15) increased plasma calprotectin levels vs controls (n=5; P=0.0001, Mann-Whitney U test). D, Illustrative images of brain heart infusion (BHI)-agar plates seeded with mesenteric lymph node (MLN) of ischemic (n=19), sham (n=11), and control (n=10) mice. Magnifications (2×) illustrate colony-forming units (CFUs) after ischemia. Quantification showed no differences between sham and controls (Figure S1D) and both groups were pooled together for comparison against ischemic mice. The proportion of mice with CFUs (Yes) in organs was higher in ischemic than sham/control (lung:*P=0.040; liver:**P=0.003; spleen:**P=0.005; mLN:**P=0.001; χ2). E, Representative brain section of sham and ischemic mice (2,3,5-triphenyltetrazolium chloride [TTC] staining). Number of bacteria CFUs (mean±SD) of ischemic (n=19) vs sham/controls (n=21) showed more CFUs in lung (*P=0.046), liver (**P=0.005), spleen (**P=0.007), and mLN (*P=0.003; Mann-Whitney U test) of ischemic mice. List of bacteria identified in ischemic mice by 16S RNA gene partial sequencing. F, Mice received antibiotics followed by oral bioluminescent Escherichia coli before ischemia (n=10) or sham operation (n=6). At 48 h, bioluminescence was detected ex vivo in the lungs of 2 ischemic but none of the sham-operated mice. BLI indicates bioluminescence imaging.
To investigate the possibility of gut bacterial translocation, we depleted mice of their own microbiota with antibiotics and colonized their gut with bioluminescent luciferase-tagged E.coli21 administered by oral gavage. The success of colonization was visualized by in vivo bioluminescence imaging. Then, we induced ischemia or sham operation and imaged the mice 48 hours postsurgery. In vivo imaging did not show any bioluminescence imaging signal in organs other than the colonized gut (Figure 1F). However, ex vivo bioluminescence imaging showed signal in the lungs of 20% of the ischemic mice and none of the sham-operated mice (Figure 1F). Although the most plausible origin is the gut, we cannot exclude the possibility that E. coli reached the respiratory tract from the oral cavity.
Stroke Reduces Transepithelial Resistance of the Gut Barrier
Dysfunction of the gut epithelial barrier could facilitate bacterial translocation from the gut lumen. Ischemia did not reduce gut permeability, as assessed by oral administration of 4-kDa fluorescein isothiocyanate–dextran in ischemic, sham, and control mice (Figure S2A and S2B). However, the results of an ex vivo preparation of the small intestine mucosa-submucosa in Ussing chambers were indicative of ischemia-induced reduction of gut barrier resistance globally combining paracellular and transcellular paths, greater permissivity to passive ion permeation, and a transient increase in electrolyte secretion as a function of stroke severity (Figure S2C). Moreover, the concentration of immunoglobulin A, which protects barrier function, decreased in the intestinal wash 2 days postischemia (Figure S2D).
Postischemic Induction of Innate Immune Responses in the Small Intestine
Gut epithelial barrier alterations could facilitate the passage of bacteria or bacterial products from the gut lumen promoting inflammation. In the ileum, ischemia upregulated the expression of genes involved in innate immune responses, like the complement pathway, whereas chemokine pathways tended to be downregulated (Figure S3A through S3C). Pathway analysis highlighted ischemia-induced upregulation of the Complement cascade and nuclear factor-κB (NF-κB) pathways. Downregulated pathways included apoptosis (Figure S3D), indicating that ischemia skews the regular balance between epithelial cell death and regeneration towards increased survival. Although ischemia did not modify the number of CX3CR1+ macrophages in the ileum (Figure S2E), expression analysis of cell type-characteristic genes suggested increased activation of macrophages versus dendritic cells, thus favoring local innate versus adaptive immune responses (Figure S3E). Altogether, these findings indicate that stroke primed the intestine for an inflammatory prosurvival innate immune response.
Ischemia Switches the Balance of Regulatory and Interferon γ+T cells
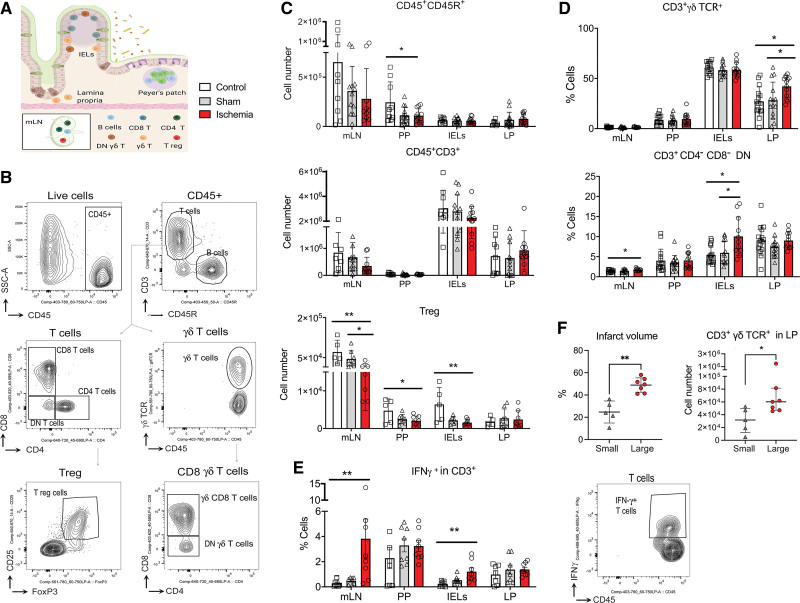
We studied the population of lymphocytes in the mLN and gut-associated lymphoid tissue after isolating intraepithelial lymphocytes, lamina propria, and Peyer patches of the small intestine of control, ischemic and sham mice 2 days postsurgery (Figure 2A) and flow cytometry analysis (Figure 2B). Ischemia reduced the number of CD45+CD45R+ B lymphocytes (Figure 2C; Figure S4A), as reported,24 and FOXP3+ regulatory T cells (Treg; Figure 2C), it increased the percentage of the innate immune lymphocytes (Figure 2D; Figure S4B and S4C) γδTCR+CD3+ T cells in lamina propria and CD3+CD4-CD8- double-negative lymphocytes in intraepithelial lymphocytes (Figure 2G). Ischemia also caused a local inflammatory T helper 1/T helper 17 shift in mLN and intraepithelial lymphocytes by increasing IFN (interferon) γ+ CD3+ (Figure 2F) and CD3-CD11b- innate lymphocytes (Figure S4D), and IL (interleukin) 17A+ γδTCR+CD3+ T cells (Figure S4E). Dichotomizing the mice into small or large lesion showed more γδTCR+CD3+ T cells in the large-infarction group (Figure 2F). We investigated whether the stroke-induced gut immune changes depended on the adrenergic stress response by treatment with propranolol, which attenuated the reduction of Tregs in Peyer patches (Figure S4F) and the CD3+CD8+ γδTCR+ cell increase in lamina propria (Figure S4F).
Figure 2.
Stroke induced a proinflamamtory shift in gut lymphocytes. A, Lymphocytes were obtained from mesenteric lymph nodes (mLN), Peyer Patches (PP), intraepithelial lymphocytes (IELs), and lamina propria (LP) from ischemic (n=12) and sham (n=14) mice 48 h postsurgery, and naive controls (n=15). Figure generated with Biorender.com. B, Gating strategy for flow cytometry analysis. C, Ischemia reduced B lymphocyte (CD45+CD45R+) number in PP (*P=0.018 vs control; 1-way ANOVA/Holm-Šídák test); CD3+ T cells showed a trend to reduction, and regulatory T cells (Tregs; CD4+CD25+forkhead box P [Foxp]3+) significantly decreased in mLN (**P=0.007 vs control; *P=0.041 vs sham; Kruskal-Wallis test/Dunn test), PP (*P=0.041 vs control), and the IELs (**P=0.006 vs control); 1-way ANOVA/Holm-Šídák test. D, Ischemia increased the % of CD3+ γδ T cell receptor (TCR)+ cells (*P=0.019 vs control and sham) in LP, and CD3+CD4-CD8- double-negative (DN) cells in mLN (*P=0.046 vs sham) and IELs (*P=0.033 vs control and sham); 1-way ANOVA/Holm-Šídák test. E, Representative cytometry plot of intracellular IFN (interferon)-γ (n=8 mice/condition). Ischemia increased vs control IFN-γ in CD45+CD3+ T cells in mLN (**P=0.003) and IELs (**P=0.002; Kruskal-Wallis test/Dunn test). F, Mice were dichotomized by the median of infarct volume in small (n=5) or large (n=7) groups (**P=0.0025, Mann-Whitney U test). Mice with larger infarcts had more CD3+γδ TCR+ cells in LP (*P=0.048; Mann-Whitney U test). Values are expressed as the mean±SD and all individual points are shown.
Ischemia Increases Primary BAs in the Liver But Reduces BA Content in the Gut
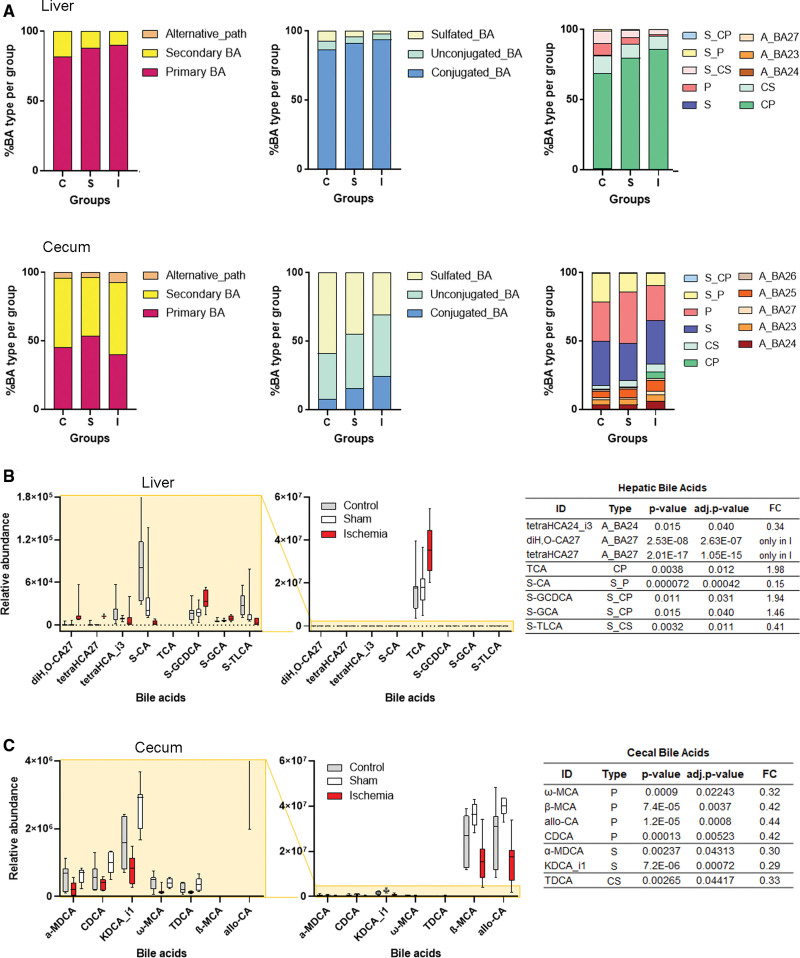
BAs are metabolic products with antibacterial activity and immune and gut barrier regulatory functions.17,25 Liver primary BAs are synthesized from cholesterol and metabolized by gut microbiota generating secondary BAs that mostly return to the liver via the portal vein. We used an untargeted metabolomics approach by liquid chromatography–mass spectrometry analysis enabling the profiling of 50 and 196 BAs in liver and cecal content, respectively.22 Ischemia increased the proportion of conjugated primary BAs in the liver (Figure 3A), notably taurocholic acid (Figure 3B), and the presence of oxysterols (diH2O-CA27; tetrahydroxycholestanoic acid) indicating abnormal metabolic processing. The BA pathway can be negatively regulated through complex mechanisms.17 However, ischemia did not cause major changes in mRNA expression of enzymes involved in BA synthesis and metabolism, except for increased Cyp27a1 mRNA (Figure S5B and S5C) in the ileum, which could favor the alternative acidic pathway, and increased steroid 5-β-reductase Akr1d1 mRNA in the liver (Figure S5A and S5C). AKR1D1 (aldo-keto-reductase family 1 member D1) participates in the alternative pathway of cholesterol metabolism and also cortisol metabolism, which is necessary to restore homeostasis following the poststroke stress response. In addition, ischemia reduced the global cecal content of secondary and primary BAs (Figure 3B). This effect can facilitate pathogenic commensal microbe overgrowth.25
Figure 3.
Stroke increases bile acids (BAs) in the liver and reduces BAs in the intestine. BA profiling by liquid chromatography–mass spectrometry–based untargeted metabolomics in liver and cecal content samples from control (n=7), sham-operated (n=8), and ischemic (n=8) mice. A, Proportions of BA according to the biosynthetic pathway, conjugation, or sulfation. B and C, Box-plots of BAs identified in liver (B) and cecum (C) BAs that were statistically significant (moderated t test) after adjustment for multiple testing (P<0.05) between ischemic and sham-operated mice. The abundance of BAs of control mice was also included in the plots, confirming that the change in the BA’s levels depends on ischemia. α-MDCA indicates α-murideoxycholic acid; β-MCA, β-muricholic acid; ω-MCA, ω-muricholic acid; A_BA, BA generated from the alternative acidic pathway; adj, adjusted; allo-CA, allo-cholic acid; C, control; CDCA, chenodeoxycholic acid; CP, conjugated primary BA; CS, conjugated secondary BA; diH,O-CA27, dihydroxy-oxocholest-enoic acid; FC, fold change; I, ischemia; KDCA_i1, ketodeoxycholic acid isomer; MCA, middle cerebral artery; P, primary BA; S, secondary BA, S_, sulfated BA; S, sham; S-CA, sulfocholic acid; S-GCA, glycocholic acid-sulfate; S-GCDCA, glycochenodeoxycholic acid-sulfate; S-TLCA, taurolithocholate-sulfate; TCA, taurocholic acid; TDCA, taurochenodeoxycholic acid; tetraHCA_i3, tetrahydroxy-cholanoic acid isomer 3; and tetraHCA27, tetrahydroxycholestanoic acid.
Brain Ischemia Increases the Abundance of Enterobacteriaceae and Other Facultative Anaerobic Bacterial Families in Feces and Cecum
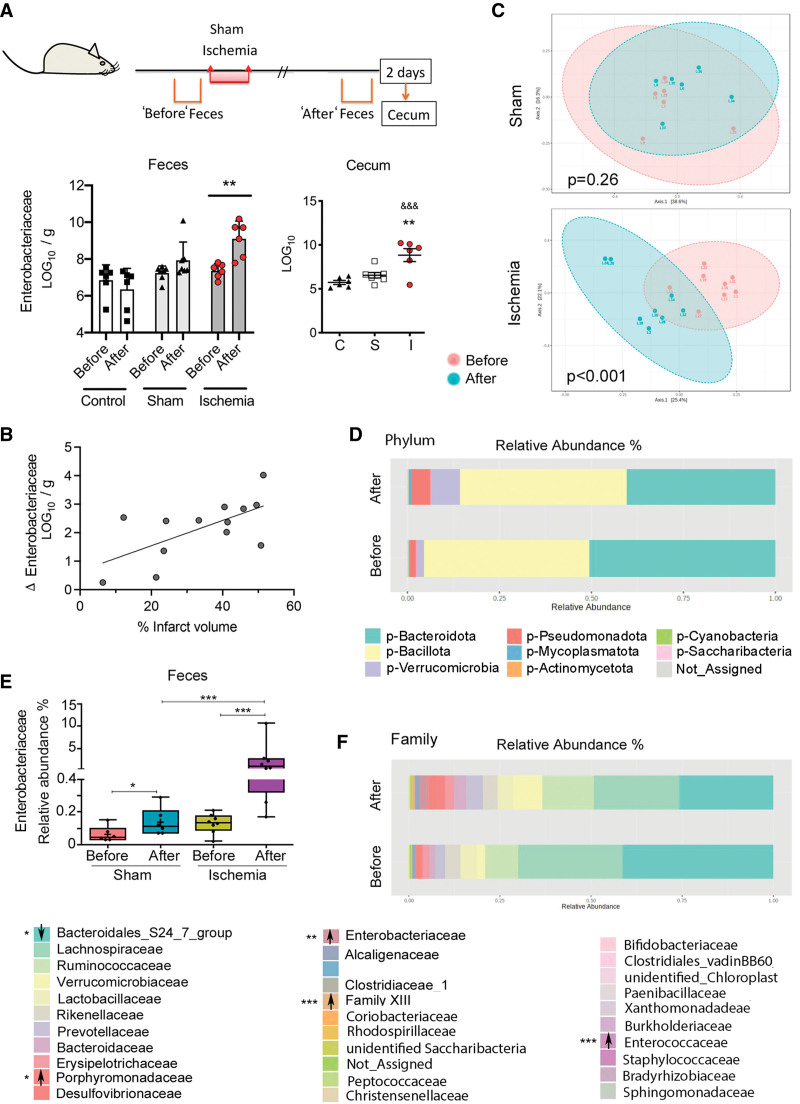
We determined the absolute levels of some microbial groups of commensal bacteria by real-time polymerase chain reaction23 in feces freshly collected before and 2 days postischemia or sham operation and twice within the same time frame in naïve controls (Figure 4A). The number of Enterobacteriaceae increased postischemia, but not sham operation or controls (Figure 4A). In contrast, we did not detect sustained and consistent changes in the other groups of bacteria analyzed (Figure S6A). Likewise, Enterobacteriaceae increased in the cecum 2 days postischemia (Figure 4A). Fecal Enterobacteriaceae overgrowth was reproduced in independent experiments, including mice from a different supplier (Figure S6B), and was positively correlated with infarct volume (Figure 4B). Gut Enterobacteriaceae expansion is considered the microbial signature of gut epithelial dysfunction and dysbiosis.26
Figure 4.
Ischemia increased opportunistic facultative anaerobes in gut microbiota. A, Feces were collected twice in the same mice, that is, before and 48 h postischemia (n=6) or sham operation (n=7), and naive mice (twice at corresponding time points; n=6). The cecum was obtained at 48 h. Quantitative polymerase chain reaction showed consistent increases in Enterobacteriaceae in feces after ischemia compared to feces before ischemia (**P=0.0015; 2-way ANOVA-repeated measures design/Sidak test), but not sham or controls. The cecum shows more Enterobacteriaceae after ischemia (**P=0.0052 vs sham; &&&P=0.0006 vs controls; 1-way ANOVA/Dunnett test). B, The Enterobacteriaceae (LOG10) change in feces after vs before ischemia in the same mice is positively correlated to the lesion volume (n=13, Pearson r=0.64, P=0.019). C, Sequencing the 16S rRNA gene from feces obtained before and after ischemia (n=8) or sham operation (n=5) using the Bray-Courtis distance shows significant changes within the ischemic (Permutational Multivariate Analysis of Variance [PERMANOVA], F value=3.5032, R2=0.20015, P<0.001), but not the sham group (PERMANOVA, F value=1.2608, r2=0.11197, P=0.26). D, Ischemia showed a trend to increase Pseudomonadota phylum. E, Sequencing data validated the ischemia-induced increase in the relative abundances of Enterobacteriaceae family and showed a small effect in sham-operated mice (2-way RM ANOVA. *P<0.05; ***P<0.0001). Data are depicted as boxplot, where the dots are individual mice and bars show median and interquartile range. F, At the Family level, ischemia not only increased Enterobacteriaceae (P<0.001, false discovery rate [FDR]<0.001), but also Clostridium family XIII (P<0.001, FDR<0.001), Enterococcaceae (P=0.001, FDR=0.007), and Porphyromonadaceae (P=0.004, FDR=0.029), whereas it decreased the highly abundant Bacteroidales_S24_7_group (P=0.003; FDR=0.024).
Metataxonomic analyses by partly sequencing the 16S rRNA gene showed changes in beta diversity between feces collected before and 2 days postischemia, but not sham operation (Figure 4C). Ischemia induced a trend (P=0.011, false discovery rate=0.106) to increase the phylum Pseudomonadota (formerly Proteobacteria), which includes Enterobacteriaceae (Figure 4D). At the family level, sequencing validated the polymerase chain reaction result showing increased Enterobacteriaceae after ischemia (Figure 4E). Analysis using false discovery rate of fecal microbial data obtained after ischemia versus before ischemia in the same mice further identified increase of other putative pathogenic and proinflammatory bacteria, such as Enterococcaceae, Porphyromonadaceae, and family XIII (Figure 4F). In contrast, ischemia reduced the abundance of Bacteroidales_S24_7_group (Figure 4G) composed of anaerobic bacteria with features of primary fermenters capable of producing the SCFAs propionate and acetate.27
Ischemia Reduces the Fecal Content of SCFA
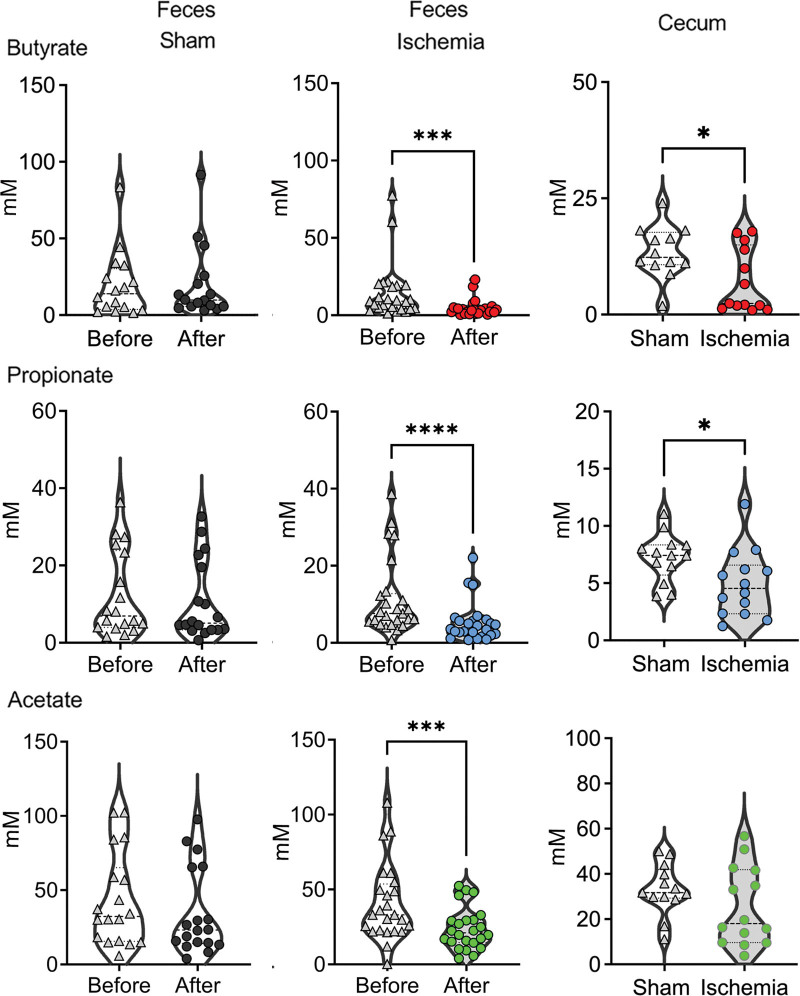
SCFA are bacterial metabolic fermentation end products with protective functions in the gut mucosa preventing inflammation and bacterial translocation. We determined the concentrations of butyrate, propionate, and acetate, which account for about 95% of all SCFAs, in the cecum 2 days postischemia or sham operation, and in the feces freshly collected before and 2 days after ischemia or sham operation. Ischemia, but not sham operation, reduced SCFA concentration in the feces (Figure 5). Moreover, butyrate and propionate also decreased in the cecum after ischemia (Figure 5).
Figure 5.
Stroke reduces gut short-chain fatty acid (SCFA). Feces were collected from the same mice before and after sham operation or ischemia, or twice in controls with 2 d difference. At day 2, mice were euthanized and the content of the cecum was collected. SCFAs were analyzed by gas chromatography–mass spectrometry. Ischemia (n=25) reduced the content of butyrate (*** P=0.0003), propionate (****P<0.0001), and acetate (***P=0.0006) in the feces vs the content before ischemia in the same mice (Wilcoxon matched-pairs signed rank test). Differences between values obtained before and after sham operation (n=7) and between the 2 control samples (n=11) were not significant so both groups were pooled together. In the cecum, the content of butyrate (*P=0.0357) and propionate (*P=0.174) was smaller in ischemic mice (n=14) vs sham/control mice (n=13; Mann-Whitney U test). Data are presented as violin plots showing all points and lines at the median and quartiles (dashed lines).
Gut Inflammation Drives Ischemia-Induced Alterations in the Gut Microbiota
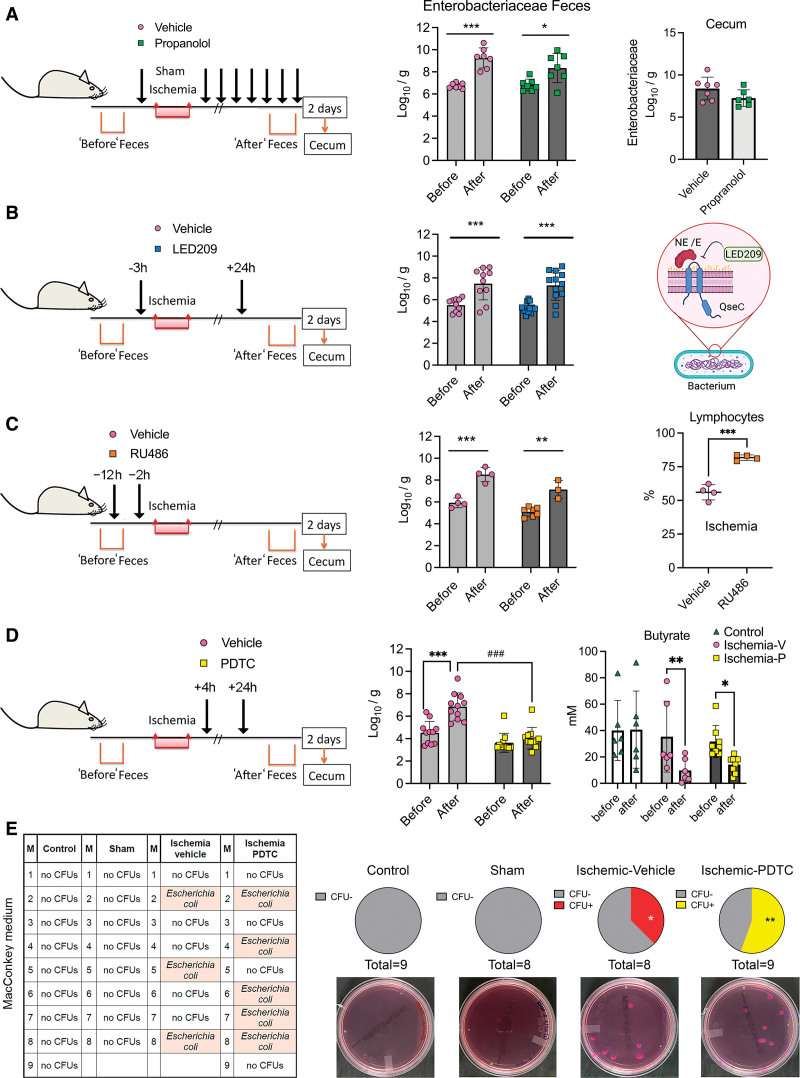
We tested whether ischemia-induced increase in Enterobacteriaceae was dependent on the β-adrenergic response. Propranolol did not prevent the ischemia-induced raise in Enterobacteriaceae in feces and cecum (Figure 6A), the reduction of gut butyrate (Figure S7A), or the changes in blood cell numbers (Figure S7A), despite preventing some of the alterations in gut lymphocyte populations (Figure S4F).
Figure 6.
The postischemic Enterobacteriaceae bloom is dependent on gut inflammation but does not mediate lung colonization with opportunistic Enterobacteria. Mice received treatments aimed to attenuate the Enterobacteriaceae expansion induced by stroke, as assessed in feces obtained before ischemia and 2 d postischemia. In some groups, we studied the cecal bacteria 2 d postischemia. Values are the mean±SD (Log10 colony forming units [CFUs]/g feces), symbols correspond to individual mice. We analyzed feces data with 2-way ANOVA-repeated measures design/Sidak multiple comparison test, and cecal data with Student t test. A, Mice were treated with propranolol (30 mg/kg; n=7) or vehicle (saline; n=7) IP immediately before ischemia, 0, 4, 8, 12, 24, 28, 32, and 36 h postischemia. The feces after ischemia showed increase in Enterobacteriaceae in the vehicle (***P=0.0009) and propranolol (*P=0.0348) groups. B, Mice received treatment with bacterial membrane-bound histidine sensor kinase quorum-sensing (QseC) inhibitor N-phenyl-4-(3-phenylthioureido)benzenesulfonamide (LED209; 16 mg/kg; n=12) or vehicle (n=10) orally 3 h before ischemia and 24 h postischemia. Fecal Enterobacteria increased after ischemia compared to before ischemia in both groups, (vehicle:**P=0.0042; LED209:***P=0.001; 2-way ANOVA-repeated measures design/Sidak test). LED209 inhibits the binding of catecholamines to the bacterial Qsec receptor (created by BioRender.com). C, Mice received RU486 (25 mg/kg; n=4) or vehicle (n=7) 12 h and 2 h before ischemia. Ischemia increased Enterobacteriaceae in feces of vehicle (***P=0.0002) and RU486 (**P=0.0093) groups. The % of lymphocytes (mean±SD) was higher after RU486 than vehicle (n=4 per group; P<0.001, Mann-Whitney U test). RU486 increased mortality (see Figure S7C). D, Mice received pyrrolidine dithiocarbamate (PDTC; 25 mg/kg) or vehicle (n=11 per group) IP 4 and 24 h postischemia. Ischemia increased Enterobacteriaceae in the vehicle (***P<0.001) but not the PDTC (P=0.5715) groups. Fecal Enterobacteriacea content after ischemia was lower in the PDTC than the vehicle group (***P<0.001). Ischemic mice, both vehicle (n=6) and PDTC (n=8) groups showed reduced butyrate vs the value before ischemia (**P=0.0063 and *P=0.0322, respectively; 2-way ANOVA-repeated-measure design/Šídák test), whereas controls (n=6) did not. E, The lungs of control (n=9), sham (n=8), or ischemic mice receiving either PDTC (n=9) or vehicle (n=8) were seeded on MacConkey agar, which selects Gram− bacteria. There was no bacterial growth in lungs of control or sham mice, but we detected colonies in ischemic mice receiving vehicle or PDTC (*P=0.027 and **P=0.006 vs sham, and *P=0.022 and **P=0.004 vs control, respectively). There were no differences between mice receiving vehicle or PDTC (P=0.228; 1-sided χ2). By matrix-assisted laser desorption/ionization-time-offlight mass spectrometry, we identified Escherichia coli. E indicates epinephrine; M, the mouse code number per group; and NE, norepinephrine.
Enterobacteriaceae bacteria express membrane-bound histidine sensor kinase quorum-sensing (QseC) system, which senses bacterial product AI-3 and host catecholamines through a bacterial receptor system resembling host α1-adrenergic receptors (Figure 6B).20 We hypothesized that Qsec may respond to host catecholamines released after brain ischemia. Oral administration of the QseC inhibitor LED20920 (Figure 6B) did not prevent the ischemia-induced expansion of Enterobacteriaceae in feces (Figure 6B) and cecum (Figure S7B) or the reduced fecal butyrate content (Figure S7B).
Ischemia-induced stress reaction triggers adrenal glucocorticoid release regulated by the hypothalamic-pituitary-adrenal axis. We studied whether a glucocorticoid-receptor antagonist (RU486) could abrogate the Enterobacteriaceae bloom induced by stroke. RU486 did not prevent the increase of Enterobacteriaceae despite attenuating stroke-induced lymphopenia (Figure 6C) and neutrophilia (Figure S7C). However, it increased mortality (Figure S7C), in agreement with the view that the adrenal corticosterone response is necessary for poststroke survival.28 These results indicate that the increase of gut facultative anaerobes after stroke is independent of immunodepression and is not attributable to the neural or hormonal arms of the stress response. However, this possibility requires further pharmacological demonstration given that we only used one dose/dosing regimen of the above drugs.
We also excluded the possibility that the Enterobacteriaceae increase after stroke was due to reduced food intake (Figure S7D). Some members of the Enterobacteriaceae family are opportunistic facultative anaerobic commensal bacteria known to overgrow in inflammatory conditions.29 We assessed the effect of postischemic intraperitoneal administration of the NF-κB inhibitor PDTC. This compound prevents nuclear NF‐κB translocation and inhibits NF‐κB p65 subunit expression. PDTC reduced p65 (Rela) and p50 (Nfkb1) mRNA in the liver (Figure S7E) but did not modify blood cell number (Figure S7F) or butyrate content (Figure 6D). PDTC did not reduce infarct volume or neurological score (Figure S7G) since we chose a dose (25 mg/kg) within the range of reported systemic anti-inflammatory actions,30 but below the minimal dose (100 mg/kg) reported to reduce ischemic damage.31 PDTC entirely prevented the Enterobacteriaceae expansion in the feces of ischemic mice (Figure 6D), suggesting the causal involvement of gut inflammation in certain microbiota imbalances induced by stroke.
Abrogation of Gut Enterobacteriaceae Expansion Does Not Prevent Bacterial Organ Colonization
PDTC-mediated abrogation of gut Enterobacteriaceae bloom could attenuate postischemic tissue colonization with opportunistic commensal bacteria. We studied bacteria colonization of the lung 2 days postischemia in mice treated with either PDTC or vehicle and controls by seeding lung tissue homogenates in brain heart infusion agar plates. The proportion of mice with lung colony-forming units was higher in ischemic mice of both treatment groups than controls (Figure S8). A total of 31 out of 59 mice showed colony-forming units growing from lung tissue. We identified the bacteria using matrix-assisted laser desorption/ionization-time-offlight mass spectrometry mass spectrometry (Figure S8). Except for 6 cases where we could not identify the bacteria, we detected opportunistic bacteria, which are common nosocomial pathogens, E. coli, Klebsiella pneumoniae, and Enterobacter cloacae complex of the Enterobacteriaceae family, E. faecalis of the Enterococcaceae family, and S. aureus of the Staphylococcaceae family. We also detected Lactobacillus murinus in one control mouse and some cases of Staphylococcus sciuri within all groups of mice. The latter bacteria are most likely contaminants from mouse skin. To verify that PDTC did not prevent the growth of Enterobacteriaceae in the lung after stroke, we studied another group of ischemic, sham, and control mice as above, but we used MacConkey agar plates specifically suited to grow Gram− bacteria facilitating Enterobacteriaceae growth. Bacteria did not grow in control and sham groups, but we detected E. coli in ischemic mice treated with either PDTC or vehicle (Figure 6E). The proportion of ischemic mice with lung bacterial growth in the PDTC and the vehicle groups was significantly higher than sham and control groups.
Discussion
We report that signs of poststroke immunodepression are accompanied by a local inflammatory shift in gut lymphoid tissues characterized by reduced Tregs and increased γδ lymphocytes and IFNγ production. Stroke also decreased the content of gut BAs and SCFAs associated with reduced fermenter bacteria in the gut microbiota. In turn, stroke promoted an expansion of facultative anaerobic commensals in the gut microbiota typically characterized by a bloom of Enterobacteriaceae that could be prevented by an anti-inflammatory treatment. However, abrogation of the gut Enterobacteriaceae bloom did not prevent lung colonization with Enterobacteriaceae after stroke, suggesting that gut dysbiosis is not critical for poststroke infection.
In organs of ischemic mice, we identified the growth of opportunistic pathogens that are facultative anaerobes of the commensal microbiota with similar features to those blooming in the gut. A recent study reported increases in gut Enterobacteriaceae after brain ischemia in mice and humans attributed to acute intestinal ischemia after stroke,15 but the origin of such putative gut ischemia is uncertain. The β-adrenergic system was involved in some of the observed gut immune alterations. However, we could not inhibit the gut Enterobacteriaceae bloom by using several pharmacological strategies intended to block the neural or humoral arms of the stress response. Although we used previously reported drug doses, our results have the limitation of lacking dose/responses studies that are necessary to fully exclude any involvement of adrenergic and corticosteroid receptors in stroke-induced gut dysbiosis.
Overgrowth of facultative anaerobic Enterobacteriaceae typically occurs under inflammatory conditions.25,29 Accordingly, an NF-κB inhibitor fully prevented the ischemia-induced enterobacterial bloom, suggesting causal involvement of local gut inflammation. Metabolic alterations can break the homeostasis of microbial populations. We report stroke-induced increase in primary BAs in the liver highlighted by taurocholic acid. Taurocholic acid can exert proinflammatory actions by signaling through sphingosine-1-phosphate receptor 2.32 Moreover, ischemia reduced global gut BA content, which could cause metabolic dysregulation and dysbiosis, colonic transit retardation and constipation, immune dysregulation, and gut Enterobacteriaceae overgrowth.17,22
Brain ischemia reduced anaerobic fermenting bacteria producing SCFAs, notably the Bacteroidales_S24_7_group,26 as reported.33 In our study, the reduction of fermenter bacteria was associated with decreased gut SCFAs, as reported in stroke patients.34 Reduction of fecal SCFAs could enhance epithelial oxygenation promoting aerobic or facultative anaerobic pathogen expansion in the gut lumen, which is a gut dysbiosis hallmark.35 The S24_7 family belongs to the Bacteroidota phylum, whose members can produce propionate and acetate, but not butyrate. A putative explanation for the reduction of butyrate could be the impairment of microbial cross-feeding mechanisms16 that would affect primary fermenters hindering butyrate production by other microbes. SCFAs have anti-inflammatory functions and are beneficial in ischemic stroke.36,37 Decrease in SCFAs can compromise gut immune homeostasis.38 In our study, SCFA reduction was accompanied by reduced Tregs and a functional switch to T helper 1/T helper 17 regulatory patterns. By manipulating the state of the gut microbiota, a previous study showed poststroke polarization of gut naive T cells towards Treg or IL-17 γδ T cells, which cause brain inflammation.11 However, elevated IFNγ and γδ cells may locally prevent bacterial infection.39
Bacteria translocation, dissemination from body mucosae, tissue expansion, and infection involves the failure of various physical and immunologic barriers. It is possible that Enterobacteriaceae bloomed not only in the gut but also other mucosae, that is, oral and respiratory tract, or perhaps the gut bacterial expansion was not critical for their translocation through the gut barrier. Furthermore, our approach targeting gut inflammation could weaken the innate immune response to fight bacteria, further exacerbating stroke-induced immunodepression. Whatever the scenario, our results suggest that stroke-induced gut dysbiosis is unlikely to be cause of poststroke infection or to have acute effects on lesion size or neurological deficits. However, stroke-induced dysbiosis may exert detrimental effects on 3-month outcome, as suggested.12,14,15 Moreover, experimental studies showed that antibiotic treatment disturbs gut microbial communities and worsens stroke outcome.40 Loss of intestinal homeostasis after antibiotic treatment could contribute to explain the failure of prophylaxis with antibiotics to improve functional outcome or mortality in stroke patients.2 Further studies should investigate whether interventions promoting restoration of gut homeostasis after stroke could improve long-term functional outcome.
Overall, we report stroke-induced immune and metabolic alterations in the gut causing local inflammation that induces a bloom of opportunistic commensals in the gut microbiota. These effects may cause further immunometabolic dysregulation and become detrimental. The study shows that an anti-inflammatory treatment after stroke can prevent the expansion of gut Enterobacteriaceae, and suggests that the growth of opportunistic commensal bacteria in the lungs or other organs after stroke does not depend on the expansion of these bacterial populations in the gut.
Article Information
Acknowledgments
We are indebted to Dr Cormac G. M. Gahan and Dr Sadeeq Ur Rahman for kindly providing click beetle red luciferase (CBR-luc)-tagged Escherichia coli. We acknowledge support of the Cytomics and Genomics facilities of Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS). Part of the work was performed at Center de Recerca Biomèdica Cellex. The Centres de Recerca de Catalunya (CERCA) Program of Generalitat de Catalunya supports IDIBAPS.
Sources of Funding
Funded by Fundació la Marató de TV3 (ref. 201723-30-31-32) to Drs Planas, Urra, and Sancho; the Ministerio de Ciencia e Innovación (MICINN)/AEI/10.13039/501100011033 and European Regional Development Fund (ERDF) A way of making Europe by the European Union (PID2020-113202RB-I00 to Dr Planas); CSIC Interdisciplinary Thematic Platform Plataforma Temática Interdisciplinar (PTI)+Neuro-Aging of the Consejo Superior de Investigaciones Científicas (Dr Planas) funded M. Gallizioli. The work of Instituto de Productos Lácteos (IPLA)-Consejo Superior de Investigaciones Científicas (CSIC) group was partly financed by grant AYUD/2021/ 50981 from Principality of Asturias. The work of Institut de Química Avançada de Catalunya (IQAC)-CSIC was financed by Ministerio de Economía y Competitividad (AGL2017-83599-R) and MICINN (PID2020-117009RB-I00). Dr Díaz-Marugan was funded by La Caixa Foundation (ID 100010434; code LCF/BQ/DE16/11570021). Dr Arboleya received a postdoctoral Juan de la Cierva contract (MICINN, Ref. IJCI-2017-32156). Work in Dr Sancho laboratory was funded by Centro de Investigaciones Cardiovasculares (CNIC), European Union’s Horizon 2020 research and innovation program under grant agreement ERC-2016-Consolidator Grant 725091, and MICINN (PID2019-108157RB/AEI/10.13039/501100011033).
Disclosures
None.
Supplemental Material
Checklist
Supplemental Methods
Figures S1–S8
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AKR1D1
- aldo-keto-reductase family 1 member D1
- BA
- bile acids
- IFNγ
- interferon-γ
- IL-17
- interleukin-17
- LED209
- N-phenyl-4-(3-phenylthioureido)benzenesulfonamide
- PDTC
- pyrrolidine dithiocarbamate
- QseC
- bacterial membrane-bound histidine sensor kinase quorum-sensing
- SCFAs
- short-chain fatty acids
For Sources of Funding and Disclosures, see page 1886.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.123.042755.
Contributor Information
Laura Díaz-Marugan, Email: laura.diazmarugan@gmail.com.
Mattia Gallizioli, Email: mattia.gallizioli@gmail.com.
Leonardo Márquez-Kisinousky, Email: leonardo.marquez@iibb.csic.es.
Silvia Arboleya, Email: silvia.arboleya@ipla.csic.es.
Annalaura Mastrangelo, Email: annalaura.mastrangelo@cnic.es.
Francisca Ruiz-Jaén, Email: paqui.ruiz@iibb.csic.es.
Jordi Pedragosa, Email: jordi.pedragosa@iibb.csic.es.
Climent Casals, Email: climentcasals@ub.edu.
Francisco Javier Morales, Email: javiermp81@gmail.com.
Sara Ramos-Romero, Email: Sara.Traserra@uab.cat.
Sara Traserra, Email: Sara.Traserra@uab.cat.
Carles Justicia, Email: cjmfat@iibb.csic.es.
Miguel Gueimonde, Email: mgueimonde@ipla.csic.es.
Marcel Jiménez, Email: Marcel.Jimenez@uab.cat.
Josep Lluís Torres, Email: joseplluis.torres@iqac.csic.es.
Xabier Urra, Email: xurra@clinic.cat.
Ángel Chamorro, Email: ACHAMORRO@clinic.cat.
David Sancho, Email: dsancho@cnic.es.
Clara G. de los Reyes-Gavilán, Email: greyes_gavilan@ipla.csic.es.
Francesc Miró-Mur, Email: francesc.miro@vhir.org.
References
- 1.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westendorp WF, Vermeij JD, Smith CJ, Kishore AK, Hodsoll J, Kalra L, Meisel A, Chamorro A, Chang JJ, Rezaei Y, et al. Preventive antibiotic therapy in acute stroke patients: a systematic review and meta-analysis of individual patient data of randomized controlled trials. Eur Stroke J. 2021;6:385–394. doi: 10.1177/23969873211056445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38:1097–1103. doi: 10.1161/01.STR.0000258346.68966.9d [DOI] [PubMed] [Google Scholar]
- 5.Wong CH, Jenne CN, Lee WY, Léger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301 [DOI] [PubMed] [Google Scholar]
- 6.Mracsko E, Liesz A, Karcher S, Zorn M, Bari F, Veltkamp R. Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain Behav Immun. 2014;41:200–209. doi: 10.1016/j.bbi.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 7.Roth S, Cao J, Singh V, Tiedt S, Hundeshagen G, Li T, Boehme JD, Chauhan D, Zhu J, Ricci A, et al. Post-injury immunosuppression and secondary infections are caused by an AIM2 inflammasome-driven signaling cascade. Immunity. 2021;54:648–659.e8. doi: 10.1016/j.immuni.2021.02.004 [DOI] [PubMed] [Google Scholar]
- 8.Stanley D, Mason LJ, Mackin KE, Srikhanta YN, Lyras D, Prakash MD, Nurgali K, Venegas A, Hill MD, Moore RJ, et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. 2016;22:1277–1284. doi: 10.1038/nm.4194 [DOI] [PubMed] [Google Scholar]
- 9.Houlden A, Goldrick M, Brough D, Vizi ES, Lénárt N, Martinecz B, Roberts IS, Denes A. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. 2016;57:10–20. doi: 10.1016/j.bbi.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, Dichgans M, Liesz A. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. 2016;36:7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016;22:516–523. doi: 10.1038/nm.4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashiro K, Tanaka R, Urabe T, Ueno Y, Yamashiro Y, Nomoto K, Takahashi T, Tsuji H, Asahara T, Hattori N. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One. 2017;12:e0171521. doi: 10.1371/journal.pone.0171521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, Putluri N, Graf J, Bryan RM, et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol. 2018;84:23–36. doi: 10.1002/ana.25250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia GH, You C, Gao XX, Zeng XL, Zhu JJ, Xu KY, Tan C-H, Xu R-T, Wu Q-H, Zhou H-W, et al. Stroke Dysbiosis Index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front Neurol. 2019;10:397. doi: 10.3389/fneur.2019.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu K, Gao X, Xia G, Chen M, Zeng N, Wang S, You C, Tian X, Di H, Tang W, et al. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut. 2021:gutjnl-2020-323263. doi: 10.1136/gutjnl-2020-323263. [DOI] [PubMed] [Google Scholar]
- 16.Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegyi P, Maléth J, Walters JR, Hofmann AF, Keely SJ. Guts and gall: bile acids in regulation of intestinal epithelial function in health and disease. Physiol Rev. 2018;98:1983–2023. doi: 10.1152/physrev.00054.2017 [DOI] [PubMed] [Google Scholar]
- 18.Winek K, Dirnagl U, Meisel A. The gut microbiome as therapeutic target in central nervous system diseases: implications for stroke. Neurotherapeutics. 2016;13:762–774. doi: 10.1007/s13311-016-0475-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durgan DJ, Lee J, McCullough LD, Bryan RM, Jr. Examining the role of the microbiota-gut-brain axis in stroke. Stroke. 2019;50:2270–2277. doi: 10.1161/STROKEAHA.119.025140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rooks MG, Veiga P, Reeves AZ, Lavoie S, Yasuda K, Asano Y, Yoshihara K, Michaud M, Wardwell-Scott L, Gallini CA, et al. QseC inhibition as an antivirulence approach for colitis-associated bacteria. Proc Natl Acad Sci U S A. 2017;114:142–147. doi: 10.1073/pnas.1612836114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ur Rahman S, Stanton M, Casey PG, Spagnuolo A, Bensi G, Hill C, Francis KP, Tangney M, Gahan CGM. Development of a click beetle luciferase reporter system for enhanced bioluminescence imaging of listeria monocytogenes: analysis in cell culture and murine infection models. Front Microbiol. 2017;8:1797. doi: 10.3389/fmicb.2017.01797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez-López M, Iborra S, Conde-Garrosa R, Mastrangelo A, Danne C, Mann ER, Reid DM, Gaboriau-Routhiau V, Chaparro M, Lorenzo MP, et al. Microbiota sensing by Mincle-Syk axis in dendritic cells regulates Interleukin-17 and -22 production and promotes intestinal barrier integrity. Immunity. 2019;50:446–461.e9. doi: 10.1016/j.immuni.2018.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arboleya S, Binetti A, Salazar N, Fernández N, Solís G, Hernández-Barranco A, Margolles A, de Los Reyes-Gavilán CG, Gueimonde M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol Ecol. 2012;79:763–772. doi: 10.1111/j.1574-6941.2011.01261.x [DOI] [PubMed] [Google Scholar]
- 24.Schulte-Herbrüggen O, Quarcoo D, Meisel A, Meisel C. Differential affection of intestinal immune cell populations after cerebral ischemia in mice. Neuroimmunomodulation. 2009;16:213–218. doi: 10.1159/000205514 [DOI] [PubMed] [Google Scholar]
- 25.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes. 2013;4:382–387. doi: 10.4161/gmic.25723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 27.Ormerod KL, Wood DL, Lachner N, Gellatly SL, Daly JN, Parsons JD, Dal’Molin CGO, Palfreyman RW, Nielsen LK, Cooper MA, et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome. 2016;4:36. doi: 10.1186/s40168-016-0181-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Kim E, Beltran C, Cho S. Corticosterone-mediated body weight loss is an important catabolic process for poststroke immunity and survival. Stroke. 2019;50:2539–2546. doi: 10.1161/STROKEAHA.119.026053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010 [DOI] [PubMed] [Google Scholar]
- 30.Cuzzocrea S, Chatterjee PK, Mazzon E, Dugo L, Serraino I, Britti D, Mazzullo G, Caputi AP, Thiemermann C. Pyrrolidine dithiocarbamate attenuates the development of acute and chronic inflammation. Br J Pharmacol. 2002;135:496–510. doi: 10.1038/sj.bjp.0704463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nurmi A, Vartiainen N, Pihlaja R, Goldsteins G, Yrjänheikki J, Koistinaho J. Pyrrolidine dithiocarbamate inhibits translocation of nuclear factor kappa-B in neurons and protects against brain ischaemia with a wide therapeutic time window. J Neurochem. 2004;91:755–765. doi: 10.1111/j.1471-4159.2004.02756.x [DOI] [PubMed] [Google Scholar]
- 32.Studer E, Zhou X, Zhao R, Wang Y, Takabe K, Nagahashi M, Pandak WM, Dent P, Spiegel S, Shi R, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55:267–276. doi: 10.1002/hep.24681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benakis C, Poon C, Lane D, Brea D, Sita G, Moore J, Murphy M, Racchumi G, Iadecola C, Anrather J. Distinct commensal bacterial signature in the gut is associated with acute and long-term protection from ischemic stroke. Stroke. 2020;51:1844–1854. doi: 10.1161/STROKEAHA.120.029262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan C, Wu Q, Wang H, Gao X, Xu R, Cui Z, Zhu J, Zeng X, Zhou H, He Y, et al. Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. JPEN J Parenter Enteral Nutr. 2021;45:518–529. doi: 10.1002/jpen.1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorbara MT, Dubin K, Littmann ER, Moody TU, Fontana E, Seok R, Leiner IM, Taur Y, Peled JU, van den Brink MRM, et al. Inhibiting antibiotic-resistant Enterobacteriaceae by microbiota-mediated intracellular acidification. J Exp Med. 2019;216:84–98. doi: 10.1084/jem.20181639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadler R, Cramer JV, Heindl S, Kostidis S, Betz D, Zuurbier KR, Northoff BH, Heijink M, Goldberg MP, Plautz EJ, et al. Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J Neurosci. 2020;40:1162–1173. doi: 10.1523/JNEUROSCI.1359-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, d’Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, Hassan A, Graf J, Petrosino J, Putluri N, et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res. 2020;127:453–465. doi: 10.1161/CIRCRESAHA.119.316448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570–575. doi: 10.1126/science.aam9949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol. 2009;182:3047–3054. doi: 10.4049/jimmunol.0802705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winek K, Engel O, Koduah P, Heimesaat MM, Fischer A, Bereswill S, Dames C, Kershaw O, Gruber AD, Curato C, et al. Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke. 2016;47:1354–1363. doi: 10.1161/STROKEAHA.115.011800 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.