Abstract
To study the difference of clinical characteristics and prognostic factors from elderly patients with renal cell carcinoma (RCC), the statistical analysis was carried out based on the surveillance, epidemiology, and end results database. The relevant clinical information of 19,472 RCC patients from 2010 to 2015 were collected, and the differences of clinicopathological characteristics and survival rate was analyzed by log-rank method and Chi square test, respectively. Multivariate Cox regression model was used to explore the independent risk factors affecting the long-term survival of RCC patients. Results showed that the proportion of elderly RCC patients in the 60–64-year group in 2010 was 15.20%, but the value elevated to 18.51% in 2015, and the Chi-square test revealed the significant correlation between elderly RCC patients with gender, race, American Joint Committee on cancer stage, T stage, N stage, and M stage. The difference of survival time between the 60–69 year, 70–79 year, 80–84 year, and 85+ year group was significant, and Kaplan–Meier analysis showed a negative effects of age on survival rate of RCC patients, indicating a worsening trend with increasing age. Cox proportional hazards model analysis further confirmed that age was the important independent prognostic factor. Our study reveals that the onset age of RCC in elderly population is gradually decreasing, and the malignant degree of elderly RCC patients is increasing with age. The female elderly population could be more susceptible to RCC than male elderly population, and 85+ year population could also be cancer susceptible with a higher lymph node metastasis rate, later tumor stage, and poor prognosis, suggesting that these elderly populations should pay more attention to the RCC screening.
Keywords: prognostic factors, renal cell carcinoma, SEER database, survival analysis
1. Introduction
Renal cell carcinoma (RCC) is one of the most common malignant tumors in the urinary system, and the incidence rate of RCC was increasing year by year.[1–3] The etiology of RCC is complex, and is closely related to genetic factors, environmental factors and living habits.[4–6] Among them, age is an important factor in the pathogenesis and prognosis of RCC, and the high incidence age group was 50 to 70 years old.[7] The significant differences in clinical characteristics and prognosis between different age groups of RCC patients was observed, and the proportion of elderly RCC patients was much higher than that of young patients.[8,9] However, the age of the elderly RCC patients in current studies was with a wider range, and patients older than 60 years were not grouped. There might be some differences between 60–70 years old and 70–80 years old, and their clinicopathological characteristics and prognosis may also be different, indicating that age might be an important factor in elderly cancer patients.[10] Further study on the clinicopathological characteristics and prognosis of elderly RCC patients in different age groups may be helpful for the prevention, diagnosis and individualized treatment of elderly RCC patients. Moreover, the sample size in most reports focusing on the age factor of RCC were relatively small, resulting that conclusions might be unreliable and controversial. Therefore, a study with large sample on the clinicopathological characteristics and prognostic factors of elder RCC patients was necessary.
Based on the Surveillance, Epidemiology, and End Results database to collect the clinical data of RCC patients from 2010 to 2015, differences of clinical characteristics of elderly RCC patients in different age groups was analyzed to explore the incidence trend and possible prognostic factors. The present study may provide theoretical support for the prevention, clinical diagnosis and precision treatment of elderly RCC patients.
2. Methods
2.1. Data sources
Using the Surveillance, Epidemiology, and End Results database, the detailed clinicopathological parameters of patients with pathologically diagnosed RCC from 2010 to 2015 were collected.
2.2. Inclusion criteria
Inclusion criteria were as follows: The pathological diagnosis was RCC; the age of the patient was more than 60 years; the year of diagnosis was from 2010 to 2015; the tumor type, differentiation degree, clinical stage and pathological data were complete; and the follow-up and prognosis information were complete.
2.3. Exclusion criteria
Exclusion criteria were as follows: The main causes of death were multiple tumors; carcinoma in situ; Incomplete tumor type, differentiation and stage; and incomplete follow-up information.
2.4. Specific collection information
We collected the following information: Age; gender; time of diagnosis, race, lymph node status; the 7th edition of American Joint Committee on cancer (AJCC) TNM staging system for renal cancer; and survival time of patients.
2.5. Groups
The patients were divided into 6-year groups: 60–64 year, 65–69 year, 70–74 year, 75–79 year, 80–84 year, and 85+ year. Statistical analysis and comparative study about clinicopathological differences and prognosis related factors in different age groups of elderly RCC patients were carried out.
2.6. Statistical analysis
SPSS 17.0 software (SPSS Inc., Chicago, IL) was used for statistical analysis. Chi square test was used to compare the differences of gender, race, diagnosis year, AJCC stage, T stage, N stage, and M stage in different age groups. The overall survival curve was drawn by Kaplan–Meier method, and the differences between the factors were calculated by log rank test. The significant variables of univariate analysis were introduced into Cox proportional hazards model for multivariate analysis, and the independent factors influencing the prognosis of RCC patients were obtained. The P < .05 was considered as the difference with statistical significance.
3. Results
3.1. Comparative analysis of the sociological characteristics and clinicopathological parameters of elderly RCC patients in different age groups
Finally, 19,472 cases meeting the screening criteria was collected in present study, and the clinicopathological characteristics of RCC patients could be found in additional files. As shown in Table 1, there were 4863 cases (24.97%), 5025 cases (25.81%), 3894 cases (20.00%), 2959 cases (15.20%), 1804 cases (9.26%), and 8135 cases (4.89%) in the 60–64 year group, 65–69 year group, 70–74 year group, 75–79 year group, 80–84 year group, and 85+ year old group, respectively.
Table 1.
Comparative analysis of sociological characteristics and clinical information of elderly patients with renal cell carcinoma in different age groups.
| Characters | Cases | 60–64 yr (%) | 65–69 yr (%) | 70–74 yr (%) | 75–79 yr (%) | 80–84 yr (%) | 85+ yr (%) | Chi-square test | |
|---|---|---|---|---|---|---|---|---|---|
| 4863 (24.97%) | 5025 (25.81%) | 3894 (20.00%) | 2959 (15.20%) | 1804 (9.26%) | 927 (4.76%) | χ2 | P | ||
| Gender | |||||||||
| Male | 12,490 (64.14%) | 3278 (67.41%) | 3280 (65.27%) | 2528 (64.92%) | 1839 (62.15%) | 1053 (58.37%) | 512 (55.23%) | 4768.175 | .000 |
| Female | 6982 (35.86%) | 1585 (32.59%) | 1745 (34.73%) | 1366 (35.08%) | 1120 (37.85%) | 751 (41.63%) | 415 (44.77%) | ||
| Race | |||||||||
| White | 16,057 (82.46%) | 3879 (79.77%) | 4074 (81.07%) | 3245 (83.33%) | 2482 (83.88%) | 1565 (86.75%) | 812 (87.59%) | 129.889 | .000 |
| Black | 2026 (10.40%) | 627 (12.89%) | 611 (12.16%) | 373 (9.58%) | 261 (8.82%) | 111 (6.15%) | 43 (4.64%) | ||
| Others | 1389 (7.13%) | 357 (7.34%) | 340 (6.77%) | 276 (7.09%) | 216 (7.30%) | 128 (7.10%) | 72 (7.77%) | ||
| Diagnosis year | |||||||||
| 2010 | 2939 (15.09%) | 739 (15.20%) | 719 (14.31%) | 570 (14.64%) | 446 (15.07%) | 310 (17.18%) | 155 (16.72%) | 41.197 | .022 |
| 2011 | 3017 (15.49%) | 779 (16.02%) | 745 (14.83%) | 597 (15.33%) | 467 (15.78%) | 304 (16.85%) | 125 (13.48%) | ||
| 2012 | 3219 (16.53%) | 809 (16.64%) | 830 (16.52%) | 613 (15.74%) | 521 (17.61%) | 284 (15.74%) | 162 (17.48%) | ||
| 2013 | 3277 (16.83%) | 786 (16.16%) | 884 (17.59%) | 660 (16.95%) | 483 (16.32%) | 308 (17.07%) | 156 (16.83%) | ||
| 2014 | 3416 (17.54%) | 859 (17.66%) | 883 (17.57%) | 679 (17.44%) | 546 (18.45%) | 286 (15.85%) | 163 (17.58%) | ||
| 2015 | 3604 (18.51%) | 891 (18.32%) | 964 (19.18%) | 775 (19.90%) | 496 (16.76%) | 312 (17.29%) | 166 (17.91%) | ||
| AJCC stage | |||||||||
| I | 11,519 (59.16%) | 3038 (62.47%) | 3106 (61.81%) | 2305 (59.19%) | 1688 (57.05%) | 942 (52.22%) | 440 (47.46%) | 181.709 | .000 |
| II | 1566 (8.04%) | 399 (8.20%) | 424 (8.44%) | 315 (8.09%) | 221 (7.47%) | 142 (7.87%) | 65 (7.01%) | ||
| III | 3694 (18.97%) | 807 (16.59%) | 859 (17.09%) | 765 (19.65%) | 624 (21.09%) | 419 (23.23%) | 220 (23.73%) | ||
| IV | 2693 (13.83%) | 619 (12.73%) | 636 (12.66%) | 509 (13.07%) | 426 (14.40%) | 301 (16.69%) | 202 (21.79%) | ||
| T stage | |||||||||
| T1 | 12,093 (62.31%) | 3151 (64.80%) | 3224 (64.16%) | 2411 (61.92%) | 1785 (60.32%) | 1023 (56.71%) | 499 (53.83%) | 135.278 | .000 |
| T2 | 2022 (10.42%) | 514 (10.57%) | 542 (10.79%) | 399 (10.25%) | 288 (9.73%) | 187 (10.37%) | 92 (9.92%) | ||
| T3 | 4604 (23.72%) | 1063 (21.86%) | 1108 (22.05%) | 958 (24.60%) | 777 (26.26%) | 429 (23.78%) | 269 (29.02%) | ||
| T4 | 690 (3.56%) | 135 (2.78%) | 151 (3.00%) | 126 (3.24%) | 109 (3.68%) | 102 (5.65%) | 67 (7.23%) | ||
| N stage | |||||||||
| N0 | 17,933 (92.18%) | 4520 (92.95%) | 4645 (92.44%) | 3617 (92.89%) | 2702 (91.31%) | 1646 (91.24%) | 803 (86.62%) | 67.217 | .000 |
| N1 | 1147 (5.90%) | 270 (5.55%) | 303 (6.03%) | 205 (5.26%) | 167 (5.64%) | 116 (6.43%) | 86 (9.28%) | ||
| N2 | 136 (0.70%) | 17 (0.35%) | 22 (0.44%) | 35 (0.90%) | 26 (0.88%) | 20 (1.11%) | 16 (1.73%) | ||
| N3 | 9 (0.05%) | 2 (0.04%) | 4 (0.08%) | 0 (0.00%) | 2 (0.07%) | 0 (0.00%) | 1 (0.11%) | ||
| NX | 229 (1.18%) | 54 (1.11%) | 51 (1.01%) | 37 (0.95%) | 44 (1.49%) | 22 (1.22%) | 21 (2.27%) | ||
| M stage | |||||||||
| MO | 17,239 (88.53%) | 4302 (88.46%) | 4474 (89.03%) | 3477 (89.29%) | 2619 (88.51%) | 1584 (87.80%) | 783 (84.47%) | 19.520 | .002 |
| M1 | 2233 (11.47%) | 561 (11.54%) | 551 (10.97%) | 417 (10.71%) | 340 (11.49%) | 220 (12.20%) | 144 (15.53%) | ||
AJCC = American Joint Committee on cancer.
Gender: 12,490 male elderly RCC patients was much more than 6980 female elderly RCC patients, and Chi square test also showed a significant correlation (P < .0001) between gender and elderly RCC patients, suggesting that the incidence rate of male was higher than that of female. With the increasing age of RCC patients, the proportion of male RCC patients decreased gradually from 67.41% of 60–64 year group to 55.23% of 80+ year group, but the proportion of the female RCC patients was increased, indicating that the female population may be more susceptible to RCC with increasing age.
Race: White population was making up the largest proportion (16,057 cases, 82.46%) in whole collected RCC cases. There were only 2026 black patients and 1389 other patients (main yellow patients), accounting for 10.40% and 7.13% of the total cases, respectively. The similar results could be found in different age groups. Race showed a significant correlation with elderly RCC patients (χ2 = 129.889, P < .0001). An increasing proportion of white RCC patients with age was observed, but the proportion of black patients was reducing.
Diagnosis year: There was 2939 cases in 2010, and then patients diagnosed with RCC increased to 3017 cases, 3219 cases, 3277 cases, 3416 cases, and 3604 cases in 2011, 2012, 2013, 2014, and 2015, respectively. The cases of elderly RCC patients were increasing year by year, and the diagnosis year was significant correlation with RCC patients (χ2 = 41.197, P = .022). In 2010 and 2011, proportion of RCC patient in the 80–84 year group was more than other group. However, the elderly group with the highest proportion of RCC patients in 2012, 2013, 2014 and 2015 was the 75–79 year group, 65–69 year group, 75–79 year group, 70–74 year group, indicating that the onset age of RCC in elderly population is gradually decreasing.
AJCC stage: There were 11,519 (59.16%), 1566 (8.04%), 3694 (18.97%), and 2693 (13.83%) elderly RCC patients in AJCC stage I stage II, stage III, and stage IV, respectively. The cases and proportion of elderly RCC patients in AJCC stage I was reducing from 3038 cases (62.47%) of the 60–64 year group, to 440 (47.46%) of the 80+ year group, but the opposite results of RCC patients were observed in AJCC stage III and IV. The lymph node metastasis and distant metastasis rate of elderly RCC patients was low, and there was only 1292 (6.64%) and 2233 (11.47%) cases with lymph node metastasis or distant metastasis, respectively. The proportion of RCC patients with lymph node metastasis in the 60–64 year group was only 5.94%, but the proportion in the 85+ year old group increased to 11.11%. Similarly, the proportion of RCC with distant metastasis increased from 11.54% in the 60–64 year group to 15.53% in the 85+ year group. A significant correlation also could be observed between RCC patients with influence factor T stage (χ2 = 135.2785, P < .0001), N stage (χ2 = 67.217, P < .0001), and M stage (χ2 = 19.52, P = .002), indicating that the malignant degree of RCC might be increasing with age.
3.2. Analysis of the factors influencing the prognosis of elderly RCC patients
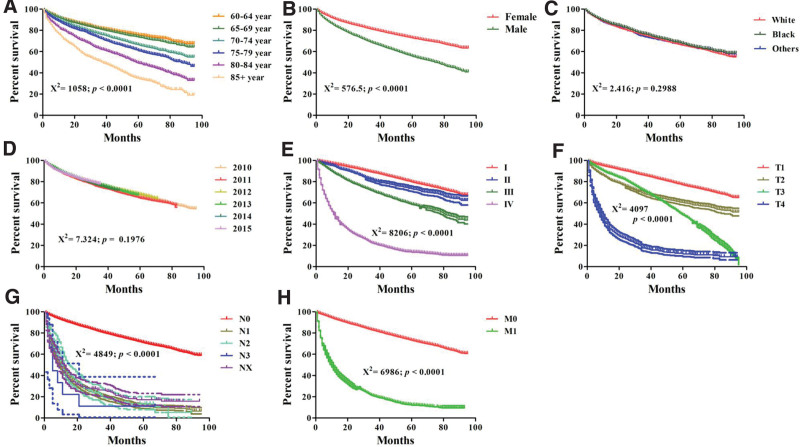
The median survival time of elderly RCC patients was 43 months, and the average survival time was 44.46 months. The 3-year and 5-year overall survival rate were 75.95% and 66.62%. Using Dunnett’s test, the significant difference of survival time between different ages was observed (Table 2), and Kaplan–Meier analysis showed a significant effect of age on survival rate of elderly RCC patients (P < .0001, χ2 = 1058, Fig. 1A). As shown in Table 2, there was no significant difference of survival time between the 60–64 year group and the 65–69 years old group, indicating a similar prognosis in both groups. The similar results could be found between the 70–74 year and the 75–79 year group. The survival time showed a negative relation with age, and the survival time was reduced from 47.68 months in the 60–64 year group to 31.32 months in the 85+ year group, suggesting a worsening trend with increasing age.
Table 2.
Survival analysis of elderly patients with renal cell carcinoma in different age groups.
| Age group | Survival time (mo) | 3-year survival rate (%) | 5-year survival rate (%) |
|---|---|---|---|
| 60–64 yr | 47.68 ± 0.36a | 81.94 | 75.60 |
| 65–69 yr | 46.68 ± 0.36a | 81.10 | 73.65 |
| 70–74 yr | 44.40 ± 0.41b | 76.44 | 66.36 |
| 75–79 yr | 43.08 ± 0.47b | 72.49 | 60.76 |
| 80–84 yr | 38.75 ± 0.62c | 62.48 | 49.13 |
| 85+ yr | 31.32 ± 0.79d | 50.95 | 34.97 |
Survival time is expressed as mean ± standard error. Values within a column having different superscript letters are significantly different.
Figure 1.
Comparison of survival rate from RCC patients in different age groups, gender group, race group, diagnosis year group, AJCC stage group, T stage group, N stage group, M stage group by Kaplan–Meier analysis. AJCC = American Joint Committee on cancer, RCC = renal cell carcinoma.
The analysis about influencing factors on the prognosis of elderly RCC patients was also carried out. Kaplan–Meier analysis showed a poor survival in the male or later stage RCC patients (Fig. 1B and E–H). The significantly positively relations between overall survival with gender, AJCC stage, T stage, N stage, M stage were found through the univariate Cox regression analyses (Table 3). Multivariate Cox regression analysis further confirmed that age, AJCC stage, T stage, N stage, and M stage were independent prognostic factors of elderly RCC patients.
Table 3.
Univariate and multivariate analysis for overall survival of elderly patients with renal cell carcinoma.
| Characters | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | ||||||
| 60–64 yr | ||||||
| 65–69 yr | 1.105 | 1.021–1.196 | .054 | 1.110 | 1.025–1.201 | .110 |
| 70–74 yr | 1.443 | 1.332–1.562 | .000 | 1.495 | 1.380–1.620 | .000 |
| 75–79 yr | 1.598 | 1.598–1.883 | .000 | 1.750 | 1.611–1.900 | .000 |
| 80–84 yr | 2.279 | 2.279–2.712 | .000 | 2.439 | 2.234–2.663 | .000 |
| 85+ yr | 3.338 | 3.338–4.075 | .000 | 3.295 | 2.978–3.646 | .000 |
| Gender | ||||||
| Male | ||||||
| Female | 1.068 | 1.014–1.125 | .012 | 1.049 | 0.996–1.105 | .073 |
| Race | ||||||
| White | ||||||
| Black | 0.936 | 0.862–1.016 | .115 | 1.204 | 1.107–1.308 | .000 |
| Others | 0.994 | 0.903–1.094 | .899 | 0.929 | 0.844–1.024 | .137 |
| Diagnosis year | ||||||
| 2010 | ||||||
| 2011 | 1.017 | 0.939–1.101 | .678 | 1.023 | 0.945–1.108 | .567 |
| 2012 | 0.930 | 0.856–1.010 | .085 | 0.920 | 0.847–0.999 | .049 |
| 2013 | 0.945 | 0.868–1.029 | .196 | 0.908 | 0.834–0.989 | .027 |
| 2014 | 0.982 | 0.899–1.073 | .691 | 0.944 | 0.864–1.031 | .200 |
| 2015 | 0.922 | 0.839–1.014 | .094 | 0.887 | 0.806–0.975 | .013 |
| AJCC stage | ||||||
| I | ||||||
| II | 1.405 | 1.264–1.561 | .000 | 1.201 | 1.011–1.426 | .038 |
| III | 2.398 | 2.245–2.562 | .000 | 2.221 | 1.958–2.519 | .000 |
| IV | 10.821 | 10.186–11.495 | .000 | 4.198 | 3.561–4.949 | .000 |
| T stage | ||||||
| T1 | ||||||
| T2 | 2.039 | 1.883–2.207 | .000 | 1.167 | 1.019–1.338 | .026 |
| T3 | 2.753 | 2.603–2.912 | .000 | 0.974 | 0.873–1.086 | .634 |
| T4 | 9.812 | 8.965–10.738 | .000 | 1.454 | 1.279–1.653 | .000 |
| N stage | ||||||
| N0 | ||||||
| N1 | 7.233 | 6.744–7.757 | .000 | 2.024 | 1.868–2.193 | .000 |
| N2 | 6.357 | 5.290–7.640 | .000 | 1.789 | 1.46–2.192 | .000 |
| N3 | 8.718 | 4.357–17.447 | .000 | 1.789 | 0.889–3.600 | .103 |
| NX | 6.467 | 5.582–7.493 | .000 | 1.596 | 1.369–1.861 | .000 |
| M stage | ||||||
| MO | ||||||
| M1 | 8.004 | 7.569–8.464 | .000 | 2.025 | 1.777–2.308 | .000 |
AJCC = American Joint Committee on cancer, CI = confidence interval, HR = hazard ratio.
As shown in Table 3, the risk of death increased gradually with age, and the values of hazard ratio (HR) was shown below: 1.110 (95%: 1.025–1.201) in 65–69 year, 1.490 (95%: 1.380–1.620) in 70–74 year, 1.750 (95%: 1.611–1.900) in 75–79 year, 2.439 (95%: 2.234–2.663) in 80–84 year, and 3.295 (95%: 2.978–3.646) in 85+ year. Compared to RCC patients in AJCC stage I, the risk of death in AJCC stage II (HR: 1.201, 95%: 1.011–1.426), III (HR: 2.221, 95%: 1.958–2.519), and IV (HR: 4.198, 95%: 3.561–4.949) patients was significantly increased. Moreover, the T stage, N stage, and M stage also elevated the risk of death of RCC patients. However, there was no significant difference between unfavorable overall survival with race (P = .2988) and diagnosis year (P = .1976), and Kaplan–Meier analysis confirmed the no-significant effect of diagnosis year and race on survival rate of RCC patients (Fig. 1C–D).
4. Discussion
RCC is the most common malignant tumors in the urinary system all over the world, and the high mortality is seriously threatening human health. Statistical results showed that the incidence rate of RCC was increasing with years,[11,12] and also showed a younger trend in elderly population. There was 3017 elderly RCC cases in 2011, but the cases increased to 3604 in 2015 (Table 1). Studies found that more than 50% of the new cases of RCC were 60-year-old people in the USA, and about 25% of the patients are over 70 years old. The increasing and serious incidence rate in elderly RCC patients suggests that the prevention, diagnosis and treatment of RCC in the elderly population should be paid more attention.[13,14] However, the lack of the large-scale clinical randomized trials and effective standardized diagnosis and treatment guidelines for the elderly RCC patients may result in that many elderly RCC patients could not get effective, scientific and reasonable diagnosis and treatment, negatively affecting the prognosis and survival of elderly RCC patients. The exploration of clinicopathological characteristics and related prognostic factors of elderly RCC patients is of great practical importance to the clinical diagnosis, treatment, and prognosis evaluation.
Denzinger et al[15] and Janke et al[9] found the significant differences in clinical characteristics and prognosis between young, middle-aged and elderly RCC patients, and showed that the age could affect the prognosis of RCC patients. However, the age range of RCC patients was wider in previous study, and the elderly RCC patients were further divided into different age groups in present study. Results showed that the cases and proportion of the 65–69 year group were higher than that of other year elderly group. Age is an important independent predictor of the prognosis,[16,17] and Kaplan–Meier analysis confirmed the significant effect of age on survival rate of elderly RCC patients (P < .0001, χ2 = 1058). The 3-year and 5-year survival rate in different groups were gradually reducing with increasing age, and the survival time showed a negative relation with age. These suggests a significantly difference in different elderly age group and confirms that age plays an important role in the prognosis of elderly RCC patients.
The demographic and sociological characteristics such as race, gender and diagnosis year were significant related with incidence rate of elderly RCC, which was consistent with the studies of Jivanji et al[18] Chi square test showed a significant correlation between gender and RCC patients (χ2 = 4768.175, P < .0001). The cases of male elderly RCC patients was much more than that of female elderly RCC patients, indicating a high incidence in the male elderly population. The positive relation between gender with the prognosis of elderly RCC patients was also observed, and survival rate of male patients was significantly lower than that of female patients. However, the proportion of male cases was gradually reducing with aging, and the female cases was contrary, indicating that the elderly female population may be more susceptible to RCC and more attention should be paid to the elderly female population.[19,20] Race is another key factor for RCC patients, and white population was making up the largest proportion in all race. However, the proportion of black patients was relatively smaller and was reducing with age, indicating an earlier age of onset and a worse prognosis for the elderly black RCC patients. Study from Palumbo et al[20] found that the prognosis of elderly black RCC patients was worse than that of white patients, which may be related to gene and socio-economic status. The diagnosis year was significant correlated with RCC patients, and the cases of elderly RCC patients were increasing during 2010 to 2015. It may be related to the growth of social population or the progress of medical technology such as RCC screening and diagnosis technology. The proportion of elderly RCC patients diagnosed in 2010 in the 80 to 84 year group was higher than that of other groups, but the group with the highest proportion was 60 to 64 year after 5 years, indicating a younger trend of RCC onset age in elderly population. The significant differences of the survival rate in different diagnosis year were not observed, suggesting the insufficiency attention or underdevelopment of diagnosis and treatment for RCC.
Clinicopathological parameters including AJCC stage, tumor size, lymph node metastasis status, and distant metastasis were found to be the important factors related with onset age and prognosis of RCC patients. As found in present study, AJCC stage I was the main pathological type, and nearly 60% of elderly RCC patients was in AJCC stage I. However, the proportion of elderly RCC patients in AJCC stage I was gradually reducing with age, but the proportion of elderly RCC patients in AJCC stage III/IV increased from 29.32% in the 60–64 year group to 45.52% in the 85+ group. Lymph node is the most important way of RCC metastasis and play an important role in RCC development. Our study further revealed that lymph node status is an independent prognostic factor of RCC and could be used as an important indicator to evaluate the survival rate and prognosis of RCC. In addition, our study also found a higher lymph node metastasis rate, later tumor stage and worse prognosis in 85+ year group, and these were consistent with the results of Selvi et al[21] and Abasse Kassim et al.[22] The curative effect of surgery was worse for the RCC patients in middle and advanced stage, and the intensified screening for the 85+ population could be necessary and helpful to the early detection of tumor.
5. Conclusion
Our study revealed the clinicopathological features of RCC in different elderly groups and found that the prognosis of RCC patients was related to the AJCC stage, tumor size, lymph node metastasis status, and distant metastasis. Age is an important independent prognostic factor and plays an important role in evaluating the prognosis of elderly RCC patients, and the prognosis of RCC patients in the 85+ year group was worse than other groups. The development of clinical guidelines is necessary for the prevention, diagnosis and treatment of elderly RCC patients, and large-scale clinical randomized trials is helpful to improve the prognosis and individualized treatment of elderly RCC patients.
Author contributions
Conceptualization: Xiaoyan Peng, Hui Sun, Jian Yang.
Data curation: Xiaoyan Peng, Hui Sun, Lingxiao Wang, Wanji Guo, Jian Yang.
Formal analysis: Hui Sun, Lingxiao Wang, Wanji Guo, Jian Yang.
Funding acquisition: Jian Yang.
Methodology: Hui Sun, Wanji Guo, Zhenxiang Zhao, Jian Yang.
Supervision: Xiaoyan Peng, Lingxiao Wang, Zhenxiang Zhao.
Writing – original draft: Xiaoyan Peng, Jian Yang.
Writing – review & editing: Hui Sun, Jian Yang.
Abbreviations:
- AJCC
- American Joint Committee on cancer
- HR
- hazard ratio
- RCC
- Renal cell carcinoma
XP and HS contributed equally to this work.
This research was supported by Natural Science Foundation of Shanxi Province (20210302123316).
All methods in this study were performed in accordance with the Declaration of Helsinki.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Peng X, Sun H, Wang L, Guo W, Zhao Z, Yang J. Analysis of clinical characteristics and prognostic factors of elderly patients with renal cell carcinoma based on the SEER database. Medicine 2023;102:25(e34069).
Contributor Information
Xiaoyan Peng, Email: 13834925683@163.com.
Hui Sun, Email: sunhui.308@163.com.
Lingxiao Wang, Email: manger-wlx@foxmail.com.
Wanji Guo, Email: 13015355888@163.com.
Zhenxiang Zhao, Email: zhaozhenxiang@sxmu.edu.cn.
References
- [1].Zhao Y, Ye G, Wang Y, et al. MiR-4461 inhibits tumorigenesis of renal cell carcinoma by targeting PPP1R3C. Cancer Biother Radiopharm. 2022;37:503–14. [DOI] [PubMed] [Google Scholar]
- [2].Sims JN, Yedjou CG, Abugri D, et al. Racial disparities and preventive measures to renal cell carcinoma. Int J Environ Res Public Health. 2018;15:1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519–30. [DOI] [PubMed] [Google Scholar]
- [4].Washio M, Mori M, Mikami K, et al. Risk factors for renal cell carcinoma in a Japanese population. Asian Pac J Cancer Prev. 2014;15:9065–70. [DOI] [PubMed] [Google Scholar]
- [5].Zuo S, Wang L, Wen Y, et al. Identification of a universal 6-lncRNA prognostic signature for three pathologic subtypes of renal cell carcinoma. J Cell Biochem. 2019;120:7375–85. [DOI] [PubMed] [Google Scholar]
- [6].Lindblad P, Wolk A, Bergström R, et al. The role of obesity and weight fluctuations in the etiology of renal cell cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 1994;3:631–9. [PubMed] [Google Scholar]
- [7].Pal DK, Maurya AK, Jana D. Comparative study of renal cell carcinoma in patients less than 40 years of age and older age patients: a retrospective single-center study. Indian J Cancer. 2018;55:297–300. [DOI] [PubMed] [Google Scholar]
- [8].Thompson RH, Ordonez MA, Iasonos A, et al. Renal cell carcinoma in young and old patients – is there a difference? J Urol. 2008;180:1262–6; discussion 1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Janke F, Füssel S, Hofmann J, et al. 732 characterization of molecular differences between renal cell carcinoma in young adult and old patients. Eur Urol Suppl. 2011;10:233–233. [Google Scholar]
- [10].Wang Y, Zhang HX, Zhang H, et al. Clinicopathological characteristics and prognosis of young patients with upper tract urethelial carcinoma. Zhonghua Bing Li Xue Za Zhi. 2021;50:90–6. [DOI] [PubMed] [Google Scholar]
- [11].Mendoza-Pérez J, Gu J, Herrera LA, et al. Genomic DNA hypomethylation and risk of renal cell carcinoma: a case-control study. Clin Cancer Res. 2016;22:2074–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zheng T, Zhu C, Bassig BA, et al. The long-term rapid increase in incidence of adenocarcinoma of the kidney in the USA, especially among younger ages. Int J Epidemiol. 2019;48:1886–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tatsugami K, Oya M, Kabu K, et al. Evaluation of efficacy and safety of sorafenib in kidney cancer patients aged 75 years and older: a propensity score-matched analysis. Br J Cancer. 2018;119:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nepple KG, Yang L, Grubb RL, 3rd, et al. Population based analysis of the increasing incidence of kidney cancer in the United States: evaluation of age specific trends from 1975 to 2006. J Urol. 2012;187:32–8. [DOI] [PubMed] [Google Scholar]
- [15].Denzinger S, Otto W, Burger M, et al. Sporadic renal cell carcinoma in young and elderly patients: are there different clinicopathological features and disease specific survival rates? World J Surg Oncol. 2007;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ito K, Asano T, Yoshii H, et al. Impact of thrombocytosis and C-reactive protein elevation on the prognosis for patients with renal cell carcinoma. Int J Urol. 2006;13:1365–70. [DOI] [PubMed] [Google Scholar]
- [17].Zhu YH, Wang X, Zhang J, et al. Low enhancement on multiphase contrast-enhanced CT images: an independent predictor of the presence of high tumor grade of clear cell renal cell carcinoma. AJR Am J Roentgenol. 2014;203:W295–300. [DOI] [PubMed] [Google Scholar]
- [18].Jivanji D, Jamieson S, Mallory C, et al. The association between race and 5-year survival in patients with clear cell renal cell carcinoma: a cohort study. Urology. 2021;148:185–91. [DOI] [PubMed] [Google Scholar]
- [19].Lughezzani G, Paciotti M, Fasulo V, et al. Gender-specific risk factors for renal cell carcinoma: a systematic review. Curr Opin Urol. 2019;29:272–8. [DOI] [PubMed] [Google Scholar]
- [20].Palumbo C, Pecoraro A, Knipper S, et al. Contemporary age-adjusted incidence and mortality rates of renal cell carcinoma: analysis according to gender, race, stage, grade, and histology. Eur Urol Focus. 2021;7:644–52. [DOI] [PubMed] [Google Scholar]
- [21].Selvi I, Demirci U, Bozdogan N, et al. The prognostic effect of immunoscore in patients with clear cell renal cell carcinoma: preliminary results. Int Urol Nephrol. 2020;52:21–34. [DOI] [PubMed] [Google Scholar]
- [22].Abasse Kassim S, Tang W, Abbas M, et al. Clinicopathologic and epidemiological characteristics of prognostic factors in post-surgical survival of colorectal cancer patients in Jiangsu Province, China. Cancer Epidemiol. 2019;62:101565. [DOI] [PubMed] [Google Scholar]