Figure 1.
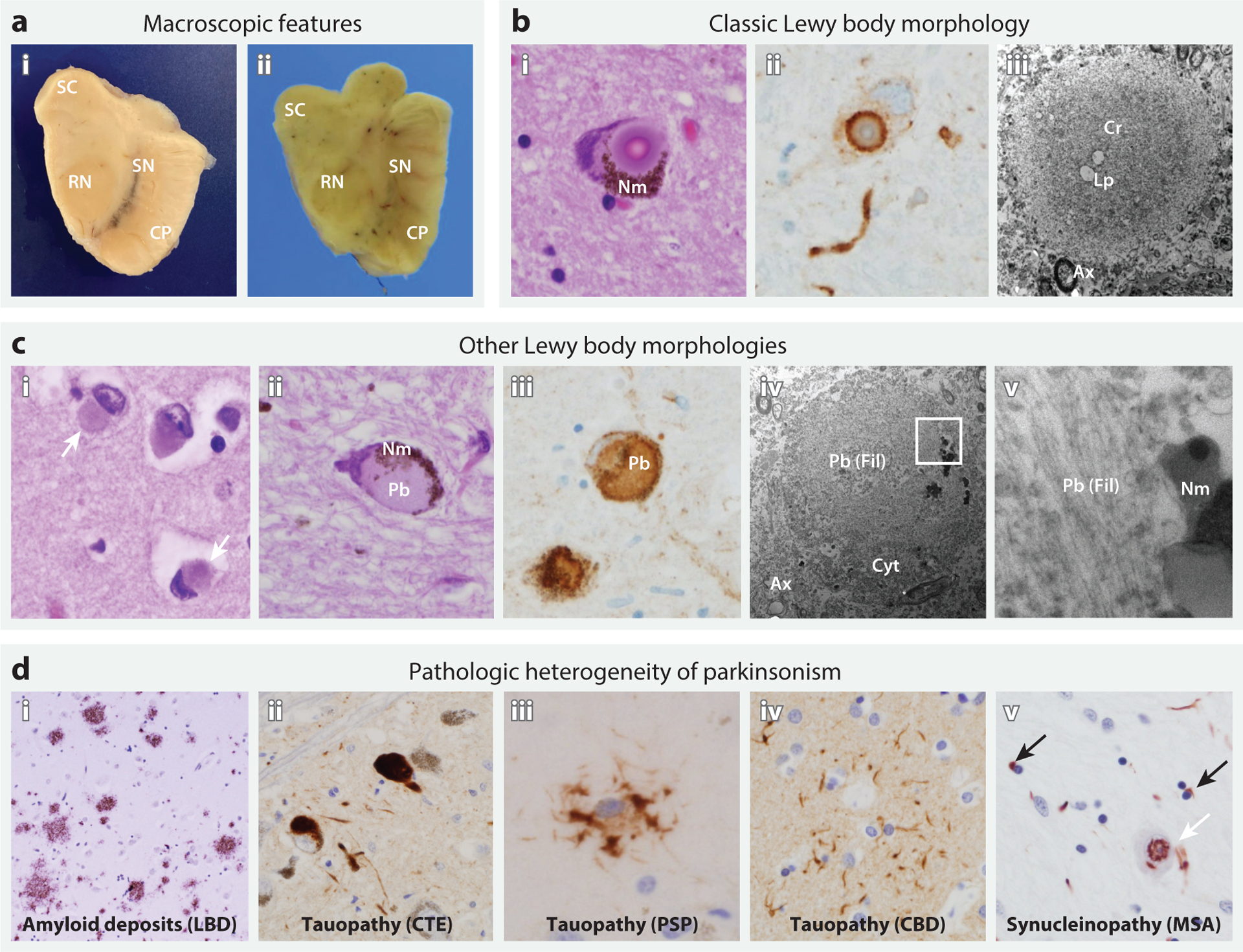
Neuropathologic features of Lewy body diseases (a,b,c) and other causes of parkinsonism (d). (a) Macroscopic appearance of the midbrain in (i) a patient without parkinsonism and (ii) a patient with Lewy body dementia (LBD), highlighting the superior colliculus (SC), red nucleus (RN), cerebral peduncle (CP), and substantia nigra (SN). In LBD, SN pigmentation is lost, whereas tectal (SC) and tegmental (RN) volumes are unaffected. Relative preservation of midbrain volume helps distinguish PD from mimics, such as progressive supranuclear palsy (PSP). (b) Classic Lewy body morphology in the SN on the basis of microscopic examination, including (i) a cytoplasmic inclusion with a dense eosinophilic core, concentric lamellae, and a pale-staining peripheral rim (hematoxylin and eosin stain). The Lewy body displaces neuromelanin (Nm) pigment within the cytoplasm. (ii) An immunostain for alpha-synuclein (αSyn) shows the dense staining of the peripheral rim of an inclusion, with less dense staining of the core. The tortuous, αSyn-positive structure below is a Lewy neurite. (iii) Electron microscopy of an extracellular Lewy body in the SN shows the characteristic dense core (Cr), here with sparse lipid droplets (Lp), a looser filamentous rim, and the adjacent neuropil with axons (Ax). (c) Other Lewy body morphologies, including (i) cortical Lewy bodies (white arrows) from temporal neocortex and (ii) pale bodies (Pb) from SN. (iii) An immunostain shows diffuse αSyn labeling of a Pb. (iv,v) Electron microscopy reveals noncompact, granulofilamentous material (Fil), entrapping and displacing cytoplasmic organelles (Cyt), and the adjacent neuropil with axons (Ax). The region enclosed by the white square is magnified (v), showing filamentous material that is sharply demarcated by Nm granules. (d) Pathologic heterogeneity of parkinsonism. Diffuse amyloid plaques are shown from a patient with LBD (i), along with characteristic pathology from several tauopathies with prominent parkinsonism, including (ii) tau-positive SN neuronal inclusions in chronic traumatic encephalopathy (CTE), (iii) a p62-positive tufted astrocyte from the striatum in PSP, and (iv) a tau-positive astrocytic plaque from the frontal cortex in corticobasal degeneration (CBD). (v) Multiple system atrophy (MSA) is characterized by αSyn-positive oligodendroglial cytoplasmic inclusions (black arrows); however, other pleomorphic, including intranuclear, inclusions are also commonly seen in neurons (white arrow).
