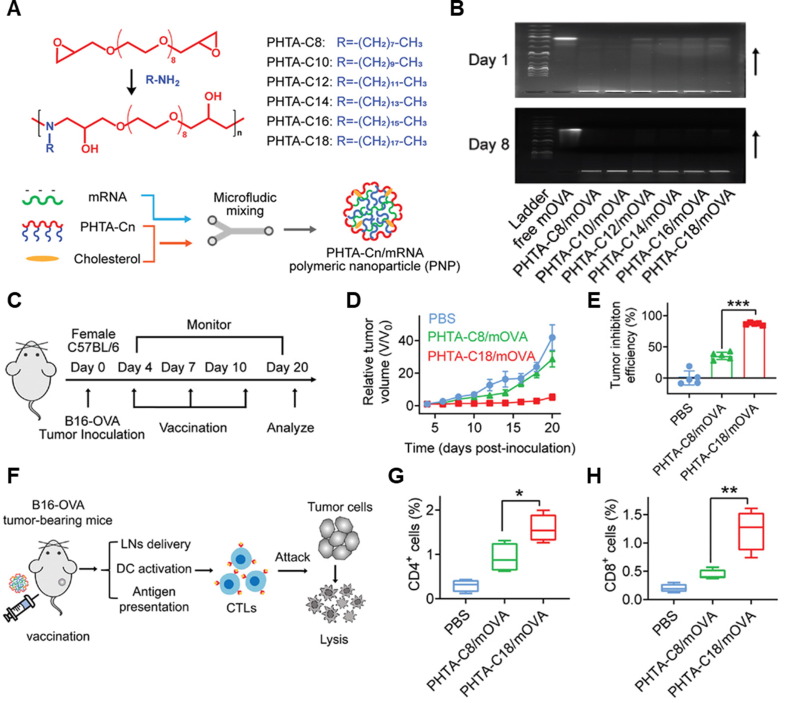
Fig. 5.
PHTA polymer-based mRNA cancer vaccine delivery system. (A) Synthetic scheme and preparation process of PHTA-Cn/mRNA nanovaccine. PHTA-Cn (n = 8, 10, 12, 14, 16, and 18) polymers were prepared via amino-epoxy polymerization. (B) Agarose gel electrophoresis images of PHTA-Cn/mRNA nanovaccine. (C) Therapeutic schedule of B16-OVA tumor-bearing mice using PHTA-C18/mRNA nanovaccine. (D) Tumor growth of PBS-, PHTA-C8/mRNA nanovaccine-, and PHTA-C18/mRNA nanovaccine-treated groups (n = 5 per group). (E) Tumor growth efficiency at the endpoint, compared with the PBS group (n = 5 per group). (F) Schematic illustration of therapeutic mechanism. Percentages of cells in B16-OVA tumor tissues on day 20: (G) CD4+ T-cells, and (H) CD8+ T-cells (n = 5 per group). Adapted with permission from reference [185].