Table 2.
Examples of application of non-viral vector-based mRNA delivery system.
| Types | Base | Structure | Application | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|---|---|
| Lipid-based delivery system | Cationic lipids | |||||
| DOPE | Activation of DC maturation and inflammatory mechanisms through interferon-α (IFNα) release by functional RNA-LPX. |
Formation of colloidally stable nanoparticulate RNA-LPX with reproducible particle size and charge. |
Immediate formation of large aggregates in near-neutral RNA-LPX, rendering them unstable. |
[105] | ||
| DOTMA | ||||||
| RNA-LPX for HPV16 vaccine. | Suitability for RNA study attributed to the cationic net charge. | Depending on the mixing ratio of lipid and RNA, there is a range in which the carrier is unstable. | [106] | |||
| A potential DC-targeting delivery system for mRNA vaccine. | Safety in vitro. |
Limitation in the in vivo delivery profile and anti-tumor efficacy. |
[107] | |||
| DOTAP | ||||||
| mRNA-based vaccine for anti-tumor immunity. |
Dual function of protecting mRNA from degradation and enhancing DC uptake. |
High level of cytotoxicity from the DOTAP/Chol/DSPE-PEG-2000 formulation. |
[108] | |||
| ePC | ||||||
| Enhancing intracellular delivery mediated by shock waves. |
Capability of delivering mRNA to diverse cancer cell types in vitro. | Cell type-dependent transfection efficiency. |
[110] |
|||
| Anionic lipids | ||||||
| 18PA, 14PA, and 18BMP |
 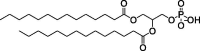 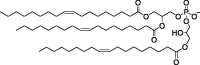
|
Selective organ targeting for tissue-specific mRNA delivery and CRISPR-Cas gene editing. | Capacity for delivering therapeutic nucleic acids. | Inability to design nanoparticles for targeted tissue delivery. | [113] | |
| Ionizable lipids | ||||||
| DLin-MC3-DMA (MC3) |
Delivery of nucleic acid-based drugs. | Facilitation of endosomal escape for efficient nucleic acid delivery to the cytosol. | Depending on the mouse strain, toxicity of LNP to the fetus may occur. | [116] | ||
| Efficient transfection of retinal pigment epithelium (RPE). | Potential for rational design of optimal cell-specific gene delivery. |
Rapid elimination of dissociated components upon entry if nanoparticles exhibit instability. |
[117] | |||
| DSPC | Effective response to virus infection. | Favorable tolerability of the system compared to alternative non-viral delivery systems. | The effectiveness of vaccines against mutated viruses may be reduced. | [121], [122] | ||
| OF-02 | Promising delivery vehicle for therapeutic mRNA delivery to the liver. | Most potent mRNA delivery vehicle reported to date in the scientific literature. |
Absence of reports on the creation of a new series of ionizable lipids specifically designed for enhancing mRNA LNP delivery in vivo. |
[127] | ||
| A2-Iso5-2DC18 and A12-Iso5-2DC18 |
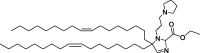 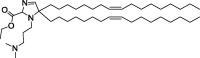
|
Optimal reduction of E7 mRNA expression, prevention of human papillomavirus, and stimulation of the STING pathway. |
Efficient delivery of mRNA. | LNP itself can trigger APC maturation. | [128] | |
| Lipid 5 | 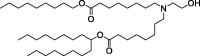 |
Improved lipid delivery and rapid elimination in non-human primates. | Good balance of delivery efficiency and pharmacokinetics. |
The efficiency of LNP delivery to organs other than the liver is unknown. |
[131] | |
| Polymer-based delivery system | PEI | 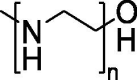 |
Efficient generation of “footprint-free” iPSCs using GO-PEI-RNA complexes for mRNA delivery. | Strong binding capacity to nucleic acids, effective uptake by cells, and excellent proton sponge effect for the endosomal release of DNA or RNA. | Significant decrease in cell viability and apparent increase in innate immune response gene expression. | [133] |
| PLGA | 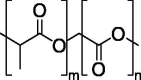 |
FDA-approved polyester type. | Carriers for DNA delivery. |
Absence of reports on intramuscular administration of PLGA-encapsulated plasmid DNA. |
[140] | |
| Poly-(β-amino ester) (PBAE) |
CAR or TCR mRNA delivery system for reprograming of circulating T cells | Less toxic than other nondegradable cationic polymers. |
A scale-up manufacturing process that can be applied clinically is essential. |
[142] | ||
| Chitosan | 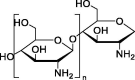 |
Promising therapeutic approach for cystic fibrosis. | Biodegradable characteristic. |
Depending on the route of administration, the efficiency of hCFTR expression varies. |
[149] | |
| Others | Gold nanoparticles | Rapid delivery of mRNA using VNB photoporation method. | Promising approach for safe and efficient intracellular mRNA delivery in cells. | Necessity to influence T cell homeostasis and therapeutic functionality. | [155] | |
| An indirect method of enhancing mRNA translation. | Enhancement of mRNA translation. |
Insufficiency of high cellular uptake and endosomal escape from endocytic vesicles. |
[157] | |||
| Silica nanoparticles | Analysis of mRNA delivery efficiency according to particle size and pore size of mesoporous SiNPs | SiNPs can be synthesized at room temperature. | The efficiency of mRNA delivery in vivo has not been confirmed. | [160] | ||
| By adding functional groups to SiNPs, new functions were added. |
As the tetrasulfide of SiNPs removed glutathione, the translation efficiency of the delivered mRNA increased. |
Cytotoxicity was observed at concentrations higher than 40 mg/ml of SiNPs. | [161] | |||
| Self-assembling mesoporous silica-cationic polymer-mRNA complex |
Tissue-specific delivery of mRNA to the pancreas and mesentery without toxicity. |
Target organs are limited. | [162] | |||
| Macrophage-targeted mRNA delivery | Silica shells protected mRNA from enzymatic degradation. |
The mechanism of macrophage-specific mRNA transfection has not been elucidated. |
[163] | |||
| Exosomes | Anti-inflammatory effects of IL-10 overexpression in atherosclerosis. | Potential to aid in disease diagnosis. | Unknown as physiological purpose of generating exosomes. | [172] | ||
| mRNA loading in exosomes via secreted after the endocytosis of LNP-mRNA system. |
Promising in vivo delivery carriers for siRNA-based therapies. |
Limitation of small size. | [173] | |||
| Potential therapeutic effects for stroke. | Low toxicity and immunogenicity. | Challenges in storage and transportation. | [174] | |||
|
Method for producing large-scale mRNA-encapsulating exosomes through cellular nanoporation. |
Favourable pharmacokinetic and immunological properties. |
Low yield observed when incorporating particularly large mRNA. |
[175] | |||
