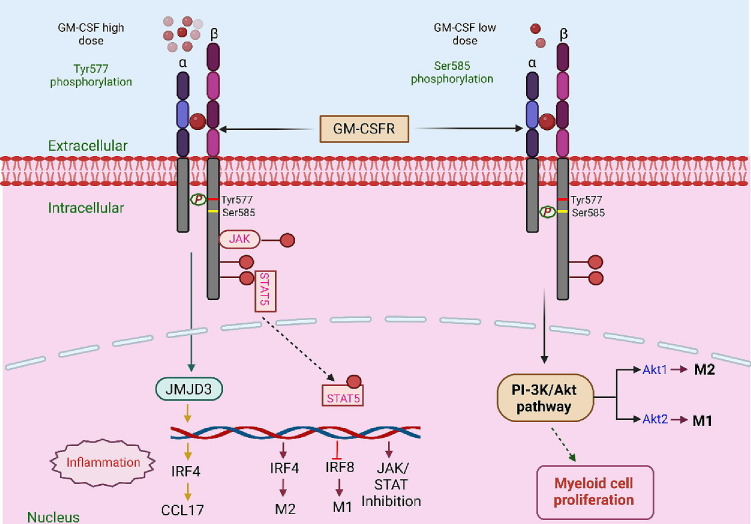
Figure 3.
Concentration dependent role of GM-CSF in cytokine storm: GM-CSF has the potential to function as both a regulatory cytokine and a proinflammatory cytokine depending on its concentration. Lower doses of GM-CSF may promote the tolerogenesis of myeloid cells, which is essential in maintaining the balance of regulatory T-cells. However, higher doses of GM-CSF induce myeloproliferation, resulting in long-lasting immunological responses. The biological activity and signaling of GM-CSF are mediated by means of attaching to the cell surface receptors of GM-CSF. The GM-CSF receptor (GM-CSF-R) represents a heterodimer comprising a GM-CSF-Rα-chain, involved in ligand binding, and a GM-CSF-Rβ -chain, involved in signal transduction. The receptor-cytokine binding could induce several cellular responses, including tyrosine phosphorylation of the β-chain and other intracellular substrates along with activation of Janus kinase 2 (JAK2)/signal transducer and activator of transcription 5 (STAT5), a mitogen-activated protein (MAP) kinase and RAS-Raf signaling pathways. The conserved motif in GM-CSF-R comprises a tyrosine (Tyr577) and serine (Ser585) residue. This motif serves as a binary switch and independently regulates multiple biological functions in a dose-dependent manner. At lower GM-CSF concentrations, signaling occurs through the Ser585 phosphorylation, which activates the PI-3 kinase pathway, thus resulting in the survival of myeloid cells. However, at higher GM-CSF levels, Tyr577 phosphorylation occurs, leading to cell survival, growth and differentiation and functional activation of signaling cascades such as JAK2/STAT5, RAS/MAPK and phosphoinositide 3-kinase (PI3K)-Akt pathway pathways. Both these processes are mutually exclusive and occur independently of each other. GM-CSF can also drive the production of CCL17 via up-regulating the expression of the IFN regulatory factor 4-dependent (IRF4-dependent) pathway by enhancing the activity of Jumonji domain-containing protein D3 (JMJD3) demethylase.