Abstract
Background:
Human social behavior is modulated by oxytocin (OT). Intranasal administration of OT (IN-OT) is a noninvasive route shown to elicit changes in the autonomic nervous system (ANS) activity; however, IN-OT’s effect on the temporal profile of ANS activity at rest is yet to be described.
Aims:
We aimed to describe the temporal profile of IN-OT at six 10-min time windows from 15- to 100-min post-administration in 20 male participants at rest while continuously recording their pupillary in an eyes-open condition and cardiac activity in eyes-open and eyes-closed conditions.
Methods:
We used a double-blind, placebo-controlled, within-subjects design study where we extracted two proxies of parasympathetic nervous system (PNS) activity: high-frequency heart rate variability (HF-HRV) and pupillary unrest index (PUI); and a proxy of sympathetic nervous system activity: sample entropy of the pupillary unrest.
Results:
In the eyes-open condition, we found an effect of IN-OT on the proxies of PNS activity: decreased PUI in the three-time windows post-administration spanning 65–100 min, and as an exploratory finding, an increased HF-HRV in the 80–85 min time window.
Conclusions:
We suggest there is a role of OT in PNS regulation that may be consistent with OT’s currently theorized role in the facilitation of alertness and approach behavior.
Keywords: Electrocardiography, neuropeptide, pharmacodynamics, pupil unrest, resting state
Introduction
Oxytocin (OT) has increasingly gathered the interest of cognitive neuroscientists since it was shown to be implicated in social cognition and behavior in humans (Erdozain and Peñagarikano, 2020) and potentially in the elusive pathophysiology of social symptoms in psychiatric disorders such as autism spectrum disorder (Huang et al., 2021; Zhao et al., 2022), schizophrenia (Liu et al., 2019), and borderline personality disorder (Jawad et al., 2021). Highly promising early studies (Averbeck et al., 2012) gave hope that pharmacological administration of OT may mitigate social behavioral deficits in these conditions (Huang et al., 2021; Shilling and Feifel, 2016). However, there is, thus far, inconsistency in findings, variability in the methods and in the outcomes and response biomarkers measured (Winterton et al., 2021), with insufficient metanalytical evidence of improvement in clinical populations (Huang et al., 2021; Sabe et al., 2021). Intranasal OT (IN-OT) is, by far, the most frequent route of OT administration in human neuroscience studies, and we have recently summarized these studies (Zelenina et al., 2022). Overall, IN-OT’s temporal profile (i.e., across a typical neuroscience experimental session time) at rest has been characterized by OT measurement in peripheral fluids (i.e., blood plasma, saliva and urine) and central nervous system OT measurements (i.e., in cerebral spinal fluid) or activity (via blood oxygen level-dependent activation using magnetic resonance imaging (MRI), and, by us, microstates using electroencephalography (EEG) (Zelenina et al., 2022). However, to the best of our knowledge, the temporal profile of IN-OT is still unexamined in the peripheral nervous system at rest. Such examination is of crucial importance for neuroscience studies’ design and interpretation because the autonomic nervous system (ANS) activity is associated with a myriad of social cognitive processes (Jáuregui et al., 2011; Quintana et al., 2012), as we have recently shown for cognitive empathy (Cosme et al., 2021, 2022). The ANS is also more easily accessible than the central nervous system in humans. Besides, such knowledge would be useful for developing biomarkers predictive of IN-OT treatment response (Erdozain and Peñagarikano, 2020; Quintana et al., 2021; Winterton et al., 2021) in preparation for clinical trials.
The effects of OT on social cognition have been linked to both central and peripherally measured nervous system activity and integrated into hypotheses such as the social salience hypothesis (Shamay-Tsoory and Abu-Akel, 2016), the general approach-avoidance hypothesis (Harari-Dahan and Bernstein, 2014) and the allostatic hypothesis (Quintana and Guastella, 2020). Heart rate variability (HRV), for example, has been implicated in social cognition (Park and Thayer, 2014), such that an increase in HRV has been associated with motivation towards social engagement (Beffara et al., 2016). Contrarily, a reduced HRV has been found in subjects with difficulties in emotion regulation (Mather and Thayer, 2018), in autism spectrum disorders (Lory et al., 2020) and first episode psychosis (Cacciotti-Saija et al., 2018) and, while at rest, may impair emotion regulation (Park and Thayer, 2014). What is not known, currently, is how the effects of OT on HRV, and other peripheric measures, at rest, integrate into OT’s cognitive hypotheses. As a first step, in this study, we aimed to develop an understanding of the effects of OT on ANS activity at rest.
OT is produced in the paraventricular, supraoptic, and accessory magnocellular nuclei of the hypothalamus (Meyer-Lindenberg et al., 2011) with direct projections to the dorsal brain stem, which regulates cardiovascular activity (Gutkowska et al., 2014), and the amygdala, which regulates ANS response patterns, particularly heart rate (Yang et al., 2007), the heart being replete with OT receptors (Gutkowska et al., 2014). Altogether, cardiac indices are suitable to assess IN-OT effects on ANS activity, particularly the parasympathetic branch. Research has also shown that increased OT receptor gene methylation (i.e., silencing) is associated with decreased parasympathetic nervous system (PNS) activity at rest via measurement of this system’s well-known positive proxy (Shaffer and Ginsberg, 2017): high-frequency heart rate variability (HF-HRV) (Lancaster et al., 2018). High HF-HRV has been associated with increased accuracy in identifying others’ positive states, which may encourage longer and more successful social relationships, and approach behaviors (Lischke et al., 2017). At rest, increased HF-HRV has also been found to predict cooperative behavior (Beffara et al., 2016) which, in turn, has been associated with increased OT function (Dreu, 2012; Rilling et al., 2012).
Yet, the characterization of IN-OT’s impact on the ANS function is still unclear, both during cognitive tasks and at rest. So far, IN-OT’s effects on each branch of the ANS have been inconsistent. During tasks, it has been found to (1) increase PNS activity (specifically, indexed by increased HF-HRV) in a facial emotion recognition task, albeit with no effect on the sympathetic nervous system (SNS) measured by electrodermal activity (Gamer and Büchel, 2012); (2) decrease PNS activity (specifically, indexed by lowered HF-HRV) while also increasing the low frequency (LF-HRV), whose meaning is still unclear, during a mental arithmetic task (Tracy et al., 2018); and (3) have no effect on PNS (specifically via HF-HRV) but increased SNS activity indexed by decreased pre-ejection period (PEP), during a social stress task (Kubzansky et al., 2012). However, the purity of the PEP as a proxy for the SNS has since been questioned given it has been associated with many other cardiovascular factors (Krohova et al., 2017). During rest, the findings also remain inconsistent by showing that IN-OT (1) coactivates both PNS and SNS (specifically, via decreasing the nonlinear HRV parameter short-term Detrended Fluctuation Analysis (DFAα1), which has been negatively associated with activation of both branches (Tulppo et al., 2005)) during a 10-min eyes-closed seated rest (Kemp et al., 2012); (2) increases PNS activity (via heightening HF-HRV) (Kemp et al., 2012), and (only) immediately after a social stress task in another study (Kubzansky et al., 2012); (3) decreases PNS activity (specifically by decreasing the root mean square of successive differences (RMSSD)) but only in females with positive childhood rearing experiences (Schoormans et al., 2020); and (4) has no influence on resting-state ANS activity (measured by HF-HRV and LF-HRV, the interval between successive R peaks (RRI) and RMSSD) (Tracy et al., 2018). All the abovementioned resting-state studies used the commonly applied 24 IU of IN-OT and a single time window, with variable lengths, albeit overlapping at 40- to 45-min post-administration. To our knowledge, only one study attempted to characterize the temporal profile, of IN-OT on HRV, but it was task-based, which we discuss later on (Norman et al., 2011).
Pupillary oscillations also reflect ANS activity; however, it has not yet been used to help characterize the effects of IN-OT at rest. The pupil’s constriction is controlled by the sphincter muscle, innervated by the PNS, and its dilation is controlled by the dilator muscle, innervated by the SNS (Mathôt, 2018), thus, the overall pupil size is modulated by the interplay of both branches of the ANS. At rest, the pupil naturally dilates and constricts in a spasmic and rhythmic fashion (Lüdtke et al., 1998). The pupillary unrest index (PUI) (Lüdtke et al., 1998; Schumann et al., 2020) is a measure of these fluctuations’ occurrence, representing the deviation in pupil dilation at low frequencies, and has been positively associated with PNS activity (using cardiac indices as proxies such as RMSSD) and, in specific, sleepiness, but negatively with alertness (Lüdtke et al., 1998; Schumann et al., 2020), and it varies with the time of day (Danker-Hopfe et al., 2001). On the other hand, sample entropy (SampEn) is a measure of the pupillary unrest’s complexity (Richman and Moorman, 2000) and has been positively associated with SNS activity (as measured by skin conductance indices) (Schumann et al., 2020). In terms of task-based research, two studies have reported increases in emotional faces stimulus-induced mean pupil dilation at 40-min post-administration, one using 24 IU and another 40 IU (Leknes et al., 2013; Prehn et al., 2013). A third study found that 8 IU of IN-OT, when administered via a Breath Powered nasal device, elicits lower facial stimuli-induced pupil dilation compared to 24 IU or placebo (Mahmoud et al., 2018), which may be explained by the dose-effect inverted-U-shaped curve previously observed for IN-OT (Borland et al., 2019).
In sum, the temporal profile of IN-OT’s effect on the ANS at rest remains to be examined, and its impact at a commonly reported time window of assessments of around 40 min is not known. In the present study, we aimed to assess the effect of 24 IU IN-OT on ANS activity at rest, across a large neuroscience experimental session duration, in healthy males, with a double-blind, randomized placebo-controlled cross-over design, recording their pupillary and cardiac activity at one baseline time window pre-administration and at six time windows post-administration (from 15 to 100 min). We examined the effect of IN-OT on proxies of PNS and SNS activity, in each time window, using electrocardiography (ECG) and pupillometry signals: two positive proxies of PNS activity (HF-HRV and PUI) (Schumann et al., 2020; Shaffer and Ginsberg, 2017) and one of SNS activity (SampEn) (Schumann et al., 2020). We specifically chose HF-HRV, a frequency measure of PNS activity, in contrast to time-domain ones (e.g., RMSSD), for comparability with previous IN-OT studies (Kemp et al., 2012; Kubzansky et al., 2012; Norman et al., 2011; Schoormans et al., 2020; Tracy et al., 2018). (However, for completeness, we report other available indexes in Supplemental Material—as explained in the Methods.) Our primary hypothesis was that IN-OT would coactivate the PNS and the SNS as reflected in an increased heart rate’s HF-HRV (Kemp et al., 2012; Kubzansky et al., 2012) which is considered a robust positive proxy of PNS activity. Aiming at providing converging evidence, we also used the pupil size’s PUI and SampEn as secondary outcomes despite being more indirect (Schumann et al., 2020) and less studied proxies of PNS and SNS, respectively. Nonetheless, we predicted that they would increase with IN-OT. These predictions are based on previous (and abovementioned) two studies’ consistent reports (one at rest and the other at rest following a social stress task), with one exception (Tracy et al., 2018) of IN-OT increasing PNS and SNS activity measured by nonlinear measures of HRV, via HF-HRV and DFAα1 (Kemp et al., 2012; Kubzansky et al., 2012), while no previous pupillometry findings are available. The aim is for our findings to assist in (1) future study design regarding the selection of the optimal IN-OT neuroscientific experimental sessions length, (2) comparability between previous and future IN-OT findings using different time windows and data modalities, (3) assessing the potential usefulness of these ANS markers as IN-OT treatment response monitoring tools, and (4) advancing our understanding of the role of OT in human cognition and behavior.
Materials and methods
Participants
We recruited 20 young (Mage = 27.4; SDage = 3.88, age range = 22–34), healthy, male, Portuguese adults, through mailouts and pamphlets in the university community and online social networks. All participants were included in the analysis. Exclusion criteria were self-reported history of endocrinological, cardiovascular, or neurological disorders; substance abuse, blocked nose; consumption of cannabis within 2 weeks prior to data collection; alcohol consumption, drugs or any medication within 24 h prior; caffeine consumption or heavy physical exercise or sexual activity on the experiment day, or tobacco smoking less than 2 h prior to the experimental session. All participants gave their written informed consent and received financial compensation for their time. The study was approved by the Ethics Committee of the Lisbon Medical Academic Center (Centro Académico Médico de Lisboa (CAML)) and complied with national and European Union legislation for clinical research.
Experimental procedure
The experimental session took place in a quiet room of the CAML’s Clinical Research Centre (Centro para Investigação Clínica) at the Hospital de Santa Maria, Lisbon, Portugal. We used a double-blind (throughout data collection up to statistical analysis, inclusive), randomized placebo-controlled, cross-over design, whereby each participant took part in two sessions: one for IN-OT and another for placebo administration, in a counterbalanced order, and at the same time each day (by 2 pm). The IN-OT administration of 24 IU was via three puffs of 0.1 ml each, in each nostril, from a 40 IU ml−1 5 ml Syntocinon bottle (using the Novartis formula, batch H5148 produced by Huningue Production, France) or an identical placebo bottle (with the same ingredients, except OT, batch 170317.01 produced by VolksApotheke Schaffhausen, Switzerland), both supplied by Victoria Apotheke Zürich, Switzerland. 24 IU of IN-OT was used, as this dose is sufficient to increase central levels of OT to a functionally relevant degree (Quintana et al., 2021). OT and placebo sessions were approximately 7 days apart. Drug storage, administration, and drug blinding efficacy are further detailed in Supplemental Material.
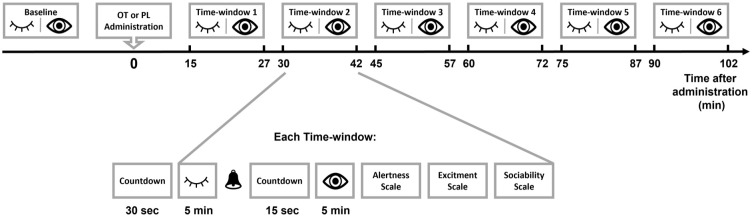
Participants spent seven time windows of 5-min eyes-closed and 5-min eyes-open (−10–0 min before administration and 15–27 min, 30–42 min, 45–57 min, 1 h to 1 h 12 min, 1 h 15 min to 1 h 27 min, 1 h 30 min to 1 h 42 min after administration) (see Figure 1) in which they were asked to stay still, avoid cognitive processes (e.g., mental arithmetic calculations), to relax, and, in the eyes-open condition, to fixate their gaze on a fixation cross at the center of a screen. At the end of each time window, the participants filled in three measures with Likert-type scales: alertness (1, alert; 5, sleepy), excitement (1, excited; 5, calm), and desire to socialize (1, desire to socialize; 5, desire to be left alone). EEG data were collected during the same experimental sessions and are reported elsewhere (Zelenina et al., 2022).
Figure 1.
The resting-state task. Neurophysiological data were recorded in eyes-closed (HRV) and eyes-open (HRV and pupil size) conditions in seven time windows, one prior to drug administration (baseline) and six post administration. Each time window was preceded by a 30-s countdown. Then followed 5 min of eyes-closed, a beep as an instruction to open eyes, a 15-s countdown, and finally five more minutes of recording. Afterwards, the participants filled in on-screen Likert-type scales for alertness, excitement and sociability. Between time windows, participants were allowed to rest until the start of the following recording period. OT: oxytocin; PL: placebo; HRV: heart rate variability.
Data acquisition and preprocessing
Pupillary activity
Participants sat comfortably with their chin supported over a chinrest to minimize head movement, at approximately 56 cm away from a Lenovo 23.8-inch screen with 1920 × 1080 resolution and 60 Hz refresh rate. At a 1000 Hz sampling rate, monocular gaze tracking and pupil size of the left eye of every participant were recorded with an SR Research EyeLink 1000 Plus which has an average accuracy of 0.15 visual angle. From the raw pupil size signal, samples 75 ms before and after blinks, as identified by the eye tracker, were converted to missing data to remove artifacts caused by partial occlusion of the eyelids (Hershman et al., 2018). Afterwards, using self-written scripts in Python v3.7.4, the signal was filtered using a third-order digital filter with a 4 Hz cutoff frequency. Missing data were linearly interpolated if it did not exceed 600 ms, as blinks longer than that are considered microsleeps (Caffier et al., 2003; Schleicher et al., 2008; Wang et al., 2011). Finally, if a 5-min time window had more than 25% of missing data, the time window was excluded from the analysis. From the fully preprocessed pupil size signal two measures were extracted, replicating Schumannet al.’s (2020) work: PUI (Lüdtke et al., 1998) and SampEn (Richman and Moorman, 2000) as each is, respectively, positively correlated to indices of PNS and SNS activity (Schumann et al., 2020). To compute the PUI, the absolute differences in the mean pupil size of consecutive segments lasting 640 ms were summed and averaged per minute (Lüdtke et al., 1998; Schumann et al., 2020). The SampEn was computed using the “pyEntropy” library (Donets et al., 2018) to input the pupil size signal down sampled to 100 Hz, embedding dimension m = 5 and tolerance level, r = 0.2 (Schumann et al., 2020).
In order to assess the possible confounding impact of the pupil foreshortening error (Hayes and Petrov, 2016) on pupil size measurements, the Euclidean distance from each sample’s location on the screen to the center (i.e., fixation cross) was subjected to the same preprocessing steps as the pupil size (see above). The main effect of drug on the Euclidean distance was not significant, F(1, 198.72) = 0.18, p = 0.671, d = 0.07, nor was the interaction with time, F(5, 191.17) = 1.12, p = 0.353. However, pairwise comparisons, per time window, indicated a difference in time window 2 (from 35 to 40 min), t(192.15) = 2.00, p = 0.047, d = 0.66, 95% confidence interval (CI) [0.01, 1.32] such that the Euclidean distance was increased under IN-OT compared to placebo.
Heart rate variability
The HRV was measured using a BIOPAC MP150 amplifier with the ECG recording module ECG100C-MRI in R wave at 1000 Hz sampling rate, gain as 1000, LP as 35 Hz and HP as 1 Hz (Biopac Systems Inc., Goleta, CA, USA) and AcqKnowledge 4.3 software. Three Ag/AgCl electrodes with 11-mm diameter (EL503 EKG , Biopac Systems Inc., Goleta, CA, USA) were placed in a Lead II disposition. The beat-to-beat RR intervals were analyzed using the Kubios Premium software (version 3.2) (Lipponen and Tarvainen, 2019; Tarvainen et al., 2014). A smoothness priors detrending method for trend removal was applied (delta = 500) with an interpolation rate of 4 Hz. After visual inspection and correction of missed or misaligned beats, artifact corrections were applied in 8.25% of all data with the very low (0.45 s) or low (0.35 s) thresholds. We used a piecewise cubic spline interpolation method (acceptance threshold 5%) for detecting RR intervals that were considered very different from the average RR interval for each participant (e.g., ectopic beats). To address our main research questions, we analyzed from the frequency domain the HF-HRV (frequency activity in the 0.15–0.40 Hz range), calculated using nonparametric Fast Fourier transformation absolute power (ms2), replicating a previous IN-OT administration at rest study (Kemp et al., 2012). As mentioned in our aims’ description, we provided in Supplemental Material the additional analysis of (1) RMSSD; (2) Kubios’ proprietary PNS and SNS indexes, the first calculated from mean RR intervals, RMSSD and Poincaré plot index S1 in normalized units, and the later calculated from mean HR, Baevky’s stress index, and Poincaré plot index S2 in normalized units; and (3) the DFAα1, particularly because it was used in a previous similar study, with statistically significant IN-OT effects (Kemp et al., 2012), albeit this measure would be more appropriately analyzed in data collected over several hours (Shaffer and Ginsberg, 2017), which we (and the previous study, in fact) have not collected.
Statistical analysis
Statistical analysis was performed in R software 3.6 (R Core Team, 2014). A linear mixed model (LMM) was run for each dependent variable (neurophysiological data: seven in total, two for pupil size-related measures and five for HRV-related measures; behavior data: three in total one for each scale) using the package “lme4” (Bates et al., 2015) with Drug session (IN-OT, placebo), Time (post-administration time window: 1, 2, 3, 4, 5, 6) and their interaction as categorical fixed factors, and participant as a random factor. For HRV, the analysis was performed separately for each condition: eyes-open and eyes-closed. Naturally, for pupil size, the analysis was only performed for the eyes-open condition. Regarding the mood scales, we have reported on the same analysis earlier in a sample differing in one subject (Zelenina et al., 2022). LMMs are suitable for datasets with missing data and inter-individual random differences (Meteyard and Davies, 2020) and allow for the inclusion of covariates of no interest that vary within subjects. As such, herein, baseline values of each drug session (i.e., the dependent variable measured at the time window prior to drug administration: time window 0), per participant, were included in the model to account for any resulting variance. For completeness, we noted that between sessions, baseline values (i.e., before the double-blind, randomized placebo-controlled administration) were significantly higher for IN-OT than placebo for PUI (t(166) = 3.91, uncorrected p < 0.001, d = 0.61, 95% CI [0.29, 0.92]), SampEn (t(172) = 4.38, uncorrected p < 0.001, d = 0.67, 95% CI [0.36, 0.99]), and HF-HRV in the eyes-open condition (t(188) = 2.35, uncorrected p = 0.020, d = 0.36, 95% CI [0.06, 0.67]). There was no significant difference for HF-HRV in the eyes-closed condition (t(186) = 0.36, uncorrected p = 0.718, d = 0.06, 95% CI [−0.25, 0.36]). The degrees of freedom and p-values were calculated using type III analysis of variance with Satterthwaite’s method. We report a measure of effect size d for LMMs, analogue to Cohen’s d, for the main effect of drug (Brysbaert and Stevens, 2018), and Cohen’s d and 95% CI for statistically significant pairwise comparisons. We considered the main effect of drug, or of the drug-by-time interaction, on the ANS measures, to be statistically significant at a Bonferroni corrected p-value of 0.05 (i.e., its uncorrected p-values were multiplied by 3, and the resulting corrected p-value was thus reported, three being the number of ANS measures we analyzed). Where such a significant effect was identified, we conducted follow-up pairwise comparisons for each time window separately. These follow-up pairwise comparisons are not corrected for multiple comparisons, as that might be overly conservative given the already a priori statistical significance of the respective omnibus test (Howell, 2009; Rothman, 1990; Saville, 1990). In cases where no omnibus test was significant (both for ANS and mood data), we have refrained from highlighting or interpreting window-based pairwise comparisons even if they survived uncorrected p < 0.05, given the higher likelihood of false positives. Thus, these should be interpreted with caution. However, in such cases, we have reported and labelled (uncorrected) p < 0.05 pairwise comparisons in Results as “Exploratory,” in order to aid potential future work with a focus on temporal-dependent IN-OT effects. All pairwise comparisons were run on estimated marginal means using the EMMEANS package from R (with degrees of freedom estimated using the Kenward-Roger method which is more precise for small samples). Since the main effect of time is not relevant to our research question, we report it only in Supplemental Material and do not interpret it.
Results
Heart rate variability
Eyes-closed
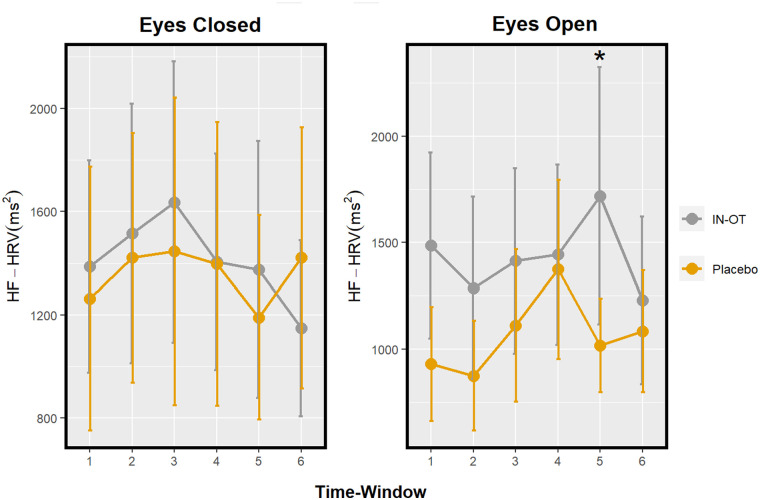
The main effect of drug on HF-HRV in eyes-closed, F(1, 184.30) = 0.18, Bonferroni corrected p > 0.999, d < 0.01, and its interaction with time, F(5, 171.94) = 0.22, Bonferroni corrected p > 0.999, was not statistically significant (Figure 2 and Table 1).
Figure 2.
Profile of HF-HRV after IN-OT in a resting-state paradigm with eyes-closed (left) and eyes-open (right) conditions. Significant pairwise comparisons (IN-OT vs placebo) at specific time windows are marked with an asterisk (*). Eyes-closed time windows: 1 = 15–20 min; 2 = 30–35 min; 3 = 45–50 min; 4 = 60–65 min; 5 = 75–80 min; and 6 = 90–95 min. Eyes-open time windows: 1 = 20–25 min; 2 = 35–40 min; 3 = 50–55 min; 4 = 65–70 min; 5 = 80–85 min; and 6 = 95–100 min. Error bars: Standard error. HF-HRV: high-frequency heart rate variability; IN-OT: intranasal oxytocin; HRV: heart rate variability.
Table 1.
Summary of the results of the effect of drug on the neurophysiological measures.
| Neurophysiological measure | Main effect of drug (IN-OT vs placebo) | Pairwise comparisons per time window (if p < 0.05) | Drug effect direction | Tentative ANS response interpretation |
|---|---|---|---|---|
| Eyes-closed | ||||
| HF-HRV | F(1, 184.30) = 0.18, Bonferroni corrected p > 0.999, d < 0.01 | – | – | – |
| Eyes-open | ||||
| HF-HRV | F(1, 175.56) = 2.11, Bonferroni corrected p = 0.444, d = 0.30 | Exploratory: TW 5: t(173.08) = 2.28, uncorrected p = 0.024, d = 0.80, 95% CI [0.10, 1.50] | IN-OT ↑ | PNS ↑ |
| PUI | F(1, 167.92) = 11.42, Bonferroni corrected p = 0.003*, d = 0.45 | TW 4: t(156.61) = 2.69, uncorrected p = 0.008, d = 0.96, 95% CI [−1.67, −0.25] | IN-OT ↓ | PNS ↓ |
| TW 5: t(158.18) = 2.25, uncorrected p = 0.026, d = 0.85, 95% CI [−1.61, −0.10] | IN-OT ↓ | PNS ↓ | ||
| TW 6: t(158.24) = 2.38, uncorrected p = 0.019, d = 0.88, 95% CI [−1.62, −0.14] | IN-OT ↓ | PNS ↓ | ||
| SampEn | F(1, 163.77) = 0.06, Bonferroni corrected p > 0.999, d = 0.54 | – | – | – |
All main effects of drug are shown; with statistically significant main effects (i.e., Bonferroni-corrected for the number of neurophysiological measures used) marked with an asterisk (*). Only follow-up pairwise comparisons surviving an uncorrected p < 0.05 are shown. Eyes-closed time windows (TWs): TW 1 =15–20 min; TW 2 = 30–35 min; TW 3 = 45–50 min; TW 4 = 60–65 min; TW 5 = 75–80 min; and TW 6 = 90–95 min. Eyes-open TWs: TW 1 = 20–25 min; TW 2 = 35–40 min; TW 3 = 50–55 min; TW 4 = 65–70 min; TW 5 = 80–85 min; and TW 6 = 95–100 min. HF-HRV: high-frequency heart rate variability; PUI: pupillary unrest index; SampEn: sample entropy; IN-OT: intranasal oxytocin; CI: confidence interval; ANS: autonomic nervous system.
Eyes-open
The main effect of drug on HF-HRV in eyes-open, F(1, 175.56) = 2.11, Bonferroni corrected p = 0.444, d = 0.30, and its interaction with time, F(5, 169.80) = 1.42, Bonferroni corrected p = 0.657, was not significant. (Exploratory pairwise comparisons in each time window indicated a significant difference in time window 5 (from 80 to 85 min), t(173.08) = 2.28, uncorrected p = 0.024, d = 0.80, 95% CI [0.10, 1.50], such that HF-HRV increased under IN-OT compared to placebo. (Figure 2 and Table 1).)
Pupillary unrest (PUI and SampEn)
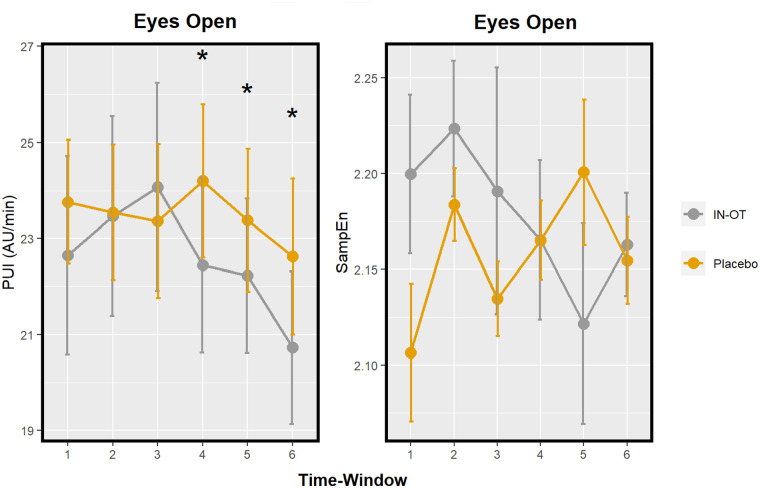
The main effect of drug on PUI was significant, F(1, 167.92) = 11.42, Bonferroni corrected p = 0.003, d = 0.45, such that PUI was decreased under IN-OT compared to placebo. Pairwise tests show this effect to be significant specifically in the last three time windows (spanning from 65 until 100 min) (respectively, t(156.61) = 2.69, uncorrected p = 0.008, d = 0.96, 95% CI [−1.67, −0.25]; t(158.18) = 2.25, uncorrected p = 0.026, d = 0.85, 95% CI [−1.61, −0.10]; t(158.24) = 2.38, uncorrected p = 0.019, d = 0.88, 95% CI [–1.62, –0.14] (Figure 3 and Table 1). A drug-by-time interaction on PUI was not significant, F(5, 155.84) = 1.60, Bonferroni corrected p = 0.492. The main effect of drug on SampEn, F(1, 163.77) = 0.06, Bonferroni corrected p > 0.999, d = 0.54, and its interaction with time were not significant, F(5, 154.48) = 1.72, Bonferroni corrected p = 0.399 (Figure 3 and Table 1).
Figure 3.
Profile of two pupil size measures after IN-OT: PUI and SampEn; in a resting-state paradigm. Significant pairwise comparisons (IN-OT vs placebo) at specific time windows are marked with an asterisk (*). Time windows: 1 = 20–25 min; 2 = 35–40 min; 3 = 50–55 min; 4 = 65–70 min; 5 = 80–85 min; and 6 = 95–100 min. Error bars: Standard error. PUI: pupillary unrest index; SampEn: sample entropy; IN-OT: intranasal oxytocin.
Behavioral and Mood scales
The main effect of drug on excitability, F(1, 220.37) = 0.76, Bonferroni corrected p > 0.999, d = 0.22, and its interaction with time, F(5, 202.49) = 1.19, Bonferroni corrected p = 0.939 were not significant. Exploratory pairwise comparisons revealed a significant difference between drugs in time window 5 (from 75 to 87 min), t(207.44) = 2.35, uncorrected p = 0.020, d = 0.76, 95% CI [0.12, 1.40], whereby excitability increased under IN-OT compared to placebo. The main effect of drug on sociability, F(1, 221) = 0.11, Bonferroni corrected p > 0.999, d = 0.54, and its interaction with time, F(5, 221) = 1.13, Bonferroni corrected p > 0.999, were not significant; nor on alertness, F(1, 206.94) < 0.01, Bonferroni corrected p > 0.999, d = 0.52; and F(5, 202.40) = 1.48, Bonferroni corrected p = 0.597, respectively.
Discussion
In this study, we aimed, for the first time, to our knowledge, to describe the temporal profile of 24 IU of IN-OT on ANS activity at rest. We included eyes-closed and eyes-open conditions and multiple time windows across a typically large neuroscience experiment duration (including a baseline assessment prior to drug administration), where we examined two positive proxies of PNS activity (HF-HRV and PUI) (Schumann et al., 2020; Shaffer and Ginsberg, 2017) and one of SNS activity (SampEn), across two data modalities (Schumann et al., 2020). Contrary to our directional hypothesis, we found an indication that IN-OT may deactivate the PNS as reflected by a decrease in PUI—starting from 65-min post-administration until the end of the last window measurement (100 min). As a secondary and exploratory finding, which needs confirmation in future studies, we found a tentative indication that IN-OT may activate the PNS since we detected it to increase HF-HRV (only) in the 80- to 85-min post-administration window. Regarding timing, we found our peak effects of IN-OT to be later than the 40-min time window researched in most studies and lasted longer than previous IN-OT studies’ usual session length (up to 90 min) (Norman et al., 2011). Lastly, we found no significant effect of IN-OT on SampEn, thus no support for an IN-OT influence on the SNS branch when measured via pupillometry. Next, we discuss these results and offer a possible explanation for the seemingly inconsistent PNS findings (between our HRV and pupillary unrest findings) which may be due to the yet unclear reliability of the pupillary unrest markers used herein and in other studies. We noted that the following interpretations should remain tentative given the early days in IN-OT and ANS association research.
The temporal profile of IN-OT effects
As abovementioned, the IN-OT effects we found both on pupillary unrest (at 65–100 min), and the exploratory finding on HF-HRV (at 80–85 min), were detected later than 40 min. Forty minutes is the starting time point which most previous studies have investigated (Gamer and Büchel, 2012; Kemp et al., 2012; Kubzansky et al., 2012; Leknes et al., 2013; Prehn et al., 2013; Schoormans et al., 2020; Tracy et al., 2018), with variable lengths, except one discussed below which used multiple time windows (Norman et al., 2011), and where they have mostly found significant IN-OT effects with two exceptions (Schoormans et al., 2020; Tracy et al., 2018). Those were specifically on HF-HRV at rest (Kemp et al., 2012; Kubzansky et al., 2012; Tracy et al., 2018) or on HF-HRV (Gamer and Büchel, 2012; Norman et al., 2011) and pupil dilation (Leknes et al., 2013; Prehn et al., 2013) using cognitive tasks. Nevertheless, none has explored IN-OT effects beyond 90 min or with pupillary unrest. Power differences may also explain the variable results since some have different designs (within- vs between-subject designs) and variable sample sizes (ranging from 21 to 173 subjects) (Gamer and Büchel, 2012; Kemp et al., 2012; Kubzansky et al., 2012; Leknes et al., 2013; Prehn et al., 2013; Schoormans et al., 2020; Tracy et al., 2018). Only one prior study has tested IN-OT effects in multiple time windows, as we have, albeit with cognitive tasks (Norman et al., 2011). Norman and colleagues (Norman et al., 2011) recorded autonomic cardiac indices during seven consecutive 15-min time windows, from a pre-administration baseline until 90 min post-administration of 20 UI of IN-OT. Direction-wise in line with our results, they found an IN-OT induced increase in HF-HRV as our exploratory finding herein, although theirs was in the 45–70-min time window, while ours was in the 80–85 min. We thus partially supported this finding in a resting-state paradigm. Crucially, this effect should not have been confounded by the significant difference we found in the baseline time window, in which HF-HRV was increased in the IN-OT group compared to placebo, given that we adjusted our statistical models for this by including the baseline data as a covariate of no interest (see Materials and methods’ section).
IN-OT decreased PUI at rest: (Unexpectedly) suggestive of PNS deactivation?
Our finding of IN-OT having an effect on pupil size at rest, that is, a medium-sized (d = 0.45) main effect of drug on PUI (but large effects, d > 0.8, at specific time windows), was such that IN-OT, unexpectedly, decreased PUI from 65 min until 100 min post-administration. This was our most statistically significant finding and most novel, given the so far only indirect evidence available of PUI’s relationship with ANS function (i.e., that PUI had recently been positively associated with RMSSD, a temporal-domain cardiac positive index of PNS activity (Schumann et al., 2020)). Interestingly the same authors also found it to be associated with skin conductance indices, which in turn was found to be a positive proxy of SNS, rather than PNS, activity in healthy controls (Schumann et al., 2017). As such, what PUI is a proxy for, in terms of ANS function, is still unclear; thus, our pupillary unrest findings, although statistically significant, should remain well open to alternative interpretations.
On the other hand, and more substantially supported by previous evidence, PUI also increases with sleepiness and drowsiness and decreases with alertness (Lüdtke et al., 1998). This could be suggested to indicate that IN-OT (by decreasing PUI) increased our study participants’ vigilance and attentive state. (This was unaffected by time-of-day variations (Danker-Hopfe et al., 2001), as all recording sessions started at approximately 2.11 pm; as in Supplemental Material—Experimental Procedure.) Our mood findings did not point to an effect of IN-OT on alertness per se, but they did—as an exploratory finding—on the somewhat related excitability in a positive relationship. More recently, OT has been hypothesized to be associated with attention and orienting responses to external social stimuli in an interplay with the dopaminergic system (Shamay-Tsoory and Abu-Akel, 2016). As such, under the “salience hypothesis of OT,” IN-OT’s effects on PUI would not be surprising, given the association of PUI with attentive states and alertness (Lüdtke et al., 1998). The sustained increase in the attentive state under IN-OT might also be explained by the closely related “approach-withdrawal hypothesis of OT” which posits it facilitates an approach to emotionally relevant stimuli (Harari-Dahan and Bernstein, 2014). OT may serve to maintain alertness in order to promote readiness to eventually engage in approach-mediated social behaviors or readiness to eventually withdraw from social stressors (Kubzansky et al., 2012).
While PUI’s direct association with each branch of the ANS has not yet been researched, HF-HRV is one of the most robust proxies of the PNS, backed by practical and theoretical evidence (Acharya et al., 2006; Shaffer and Ginsberg, 2017). As such, although our HF-HRV significant result was found on an exploratory basis (i.e., with an increased risk of being a false positive given that the main effect of drug was not statistically significant, and the follow-up pairwise comparisons were not corrected for multiple comparisons), we herein briefly and tentatively comment on them. Our exploratory HF-HRV finding was that it increased under IN-OT at rest, in the 80- to 85-min time window, which is in the same direction as the two other IN-OT resting-state studies (Kemp et al., 2012; Kubzansky et al., 2012). Such findings suggest that IN-OT upregulates PNS and may be consistent with OT motivating approach behaviors (again, in support of the “approach-withdrawal hypothesis of OT”; Harari-Dahan and Bernstein, 2014). Alternatively, one previous study found no effect of IN-OT on HF-HRV at rest but found IN-OT to decrease HF-HRV during a mental arithmetic task (Tracy et al., 2018), suggesting that, in the presence of a stressor, OT inhibits the PNS rather than triggers it (causing an effect analogous to SNS activation; Chrousos and Gold, 1992), in order to solve the stressful situation and maintain an optimal internal state (Kemp et al., 2012; Kubzansky et al., 2012; Quintana et al., 2013); while, at rest, OT may induce relaxation and lowered anxiety (Dodhia et al., 2014). In sum, we speculate that although our HF-HRV exploratory finding seems to contradict our PUI finding, the former has directional support from previous IN-OT studies (Kemp et al., 2012; Kubzansky et al., 2012), and both might be consistent with the facilitation of alertness and preparedness for an approach behavior, given previous evidence (Baethge et al., 2019; Beffara et al., 2016; Harari-Dahan and Bernstein, 2014; Kemp et al., 2012).
Limitations
Herein we computed the Euclidean distance from each sample’s location to the center of the screen (i.e., fixation cross) and subjected this measure to the same statistical analysis of our dependent variables—to assess a, by chance, possible confounding effect of the pupil foreshortening error on our drug effect analyses (Hayes and Petrov, 2016). This was not verified, as this measure was (positively) associated with the drug effect only in the 35- to 40-min time window (eyes-open time window 2), where we report no statistically significant effects. Additionally, we recognize variable IN-OT dosages would have allowed us to improve our pharmacokinetic modeling; nevertheless, we chose the most commonly administered dosage in the literature for comparability (Zelenina et al., 2022). Although an apparent limitation, we have also not measured OT blood levels as (i) they do not necessarily reflect CNS activity and (ii) they could represent the simulation of endogenous OT release, as well as the administered OT (Martins et al., 2020) and (iii) the stress-inducing phlebotomy has noisy effects on ANS activity (Alley et al., 2019; Brown et al., 2016). We also reiterate that our choice of conducting an exploratory analysis of this dataset and thereby presenting follow-up pairwise tests without correction for multiple comparisons (even when the omnibus test was not significant) means that pairwise tests relating to HF-HRV carry an increased risk of false positivity and therefore should be interpreted with caution. In addition, power limitations may have prevented the detection of significant IN-OT effects on HF-HRV consistently across all time windows, as others have achieved with a sample size doubling ours (Norman et al., 2011). However, while the latter employed a between-subjects design, our within-subject design should have been equally powerful with a smaller sample. Indeed, like us, another study reported the effects of IN-OT on HF-HRV with approximately 20 subjects in a within-subject design (Kemp et al., 2012), while another with an increased sample size (IN-OT N = 87 and placebo N = 86) in a between-subject design, found no such effects (Schoormans et al., 2020). Overall, given the mixed literature and the early days of ANS and IN-OT research, we cannot so far exclude that the measures we used are not robust markers of IN-OT effects. Finally, our results reflect the temporal profile of IN-OT on male ANS activity and cannot be generalized to females, given evidence that OT baseline levels are 3× higher in women than in men, as measured in plasma (Marazziti et al., 2019), that menstrual cycle impacts OT levels (Mitchell et al., 1981; Salonia et al., 2005; Stock et al., 1991), and of sex-related differences in several functional effects of IN-OT (Evans et al., 2014). The same evidence was behind our choice of restricting our target population to males to increase statistical power and reduce model complexity.
Conclusions
We report herein on the temporal profile of IN-OT in the human ANS at rest using HRV and, for the first time, pupillary unrest measures. Having found OT to decrease PUI (suggesting PNS deactivation) and tentatively (as an exploratory finding) that it may increase HF-HRV (suggesting PNS activation), we speculated that both might be consistent with the facilitation of alertness and preparedness for an approach behavior. Given the early days of IN-OT and ANS association research, the interpretation of these results remains speculative. Nevertheless, we hope our findings may assist in future study design, in the investigation of the comparability between IN-OT findings across data modalities, and in the assessment of usefulness of ANS markers for IN-OT response monitoring and human social cognition understanding.
Supplemental Material
Supplemental material, sj-docx-1-jop-10.1177_02698811231158233 for Temporal profile of intranasal oxytocin in the human autonomic nervous system at rest: An electrocardiography and pupillometry study by Gonçalo Cosme, Patrícia Arriaga, Pedro J. Rosa, Mitul A. Mehta and Diana Prata in Journal of Psychopharmacology
Acknowledgments
We would like to thank the Direction of the Clinical Research Centre (Centro de Investigação Clínica) of the Lisbon Medical Academic Center (Centro Académico Médico de Lisboa), represented by Prof. Luís Costa, for allowing us to conduct the experimental sessions in its facilities. We would also like to thank Marie Zelenina, Mónica Costa, and Katja Brodmann for their contribution to participants’ recruitment and data collection and all the participants who made this study possible. We thank Jasmine Ho for proofreading the final manuscript.
Footnotes
Author contributions: GC performed pupillometry data preprocessing and statistical analyses and drafted the manuscript. PR provided advice in pupillometry data preprocessing and analysis. PA preprocessed ECG data and advised on its analysis. MAM contributed to the study design and results interpretation. DP supervised and was responsible for the overall work, from study design to write-up. All authors discussed the results and revised and agreed with the final version of the manuscript.
Data availability: Data is available upon request to the authors.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Fundação para a Ciência e Tecnologia PhD fellowship (SFRH/BD/148088/2019) to GC. By the European Commission Seventh Framework Programme Marie Curie Career Integration Grant (FP7-PEOPLE-2013-CIG-631952), the 2016 Bial Foundation Psychophysiology Grant grant—(Ref. 292/16), and FCT’s (IF/00787/2014), (LISBOA-01-0145-FEDER-030907) and (UIDB/00645/2020) grants to DP. DP was also the recipient of the FCT (DSAIPA/DS/0065/2018) grant and the iMM Lisboa Director’s Fund Breakthrough Idea Grant 2016 and is co-founder and shareholder of the neuroimaging research services company NeuroPsyAI, Ltd.
ORCID iDs: Gonçalo Cosme  https://orcid.org/0000-0003-4324-8770
https://orcid.org/0000-0003-4324-8770
Diana Prata  https://orcid.org/0000-0002-4051-022X
https://orcid.org/0000-0002-4051-022X
Supplemental material: Supplemental material for this article is available online.
References
- Acharya UR, Joseph KP, Kannathal N, et al. (2006) Heart rate variability: a review. Med Biol Eng Comput 44: 1031–1051. DOI: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- Alley J, Diamond LM, Lipschitz DL, et al. (2019) Associations between oxytocin and cortisol reactivity and recovery in response to psychological stress and sexual arousal. Psychoneuroendocrinol 106: 47–56. DOI: 10.1016/J.PSYNEUEN.2019.03.031. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Bobin T, Evans S, et al. (2012) Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med 42: 259–266. DOI: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baethge A, Vahle-Hinz T, Rigotti T. (2019) Coworker support and its relationship to allostasis during a workday: a diary study on trajectories of heart rate variability during work. J Appl Psychol 105: 506–526. DOI: 10.1037/apl0000445. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker BM, et al. (2015) Fitting linear mixed-effects models using lme4. J Stat Software 67: 1–48. DOI: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Beffara B, Bret AG, Vermeulen N, et al. (2016) Resting high frequency heart rate variability selectively predicts cooperative behavior. Physiol Behav 164: 417–428. DOI: 10.1016/J.PHYSBEH.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Borland JM, Rilling JK, Frantz KJ, et al. (2019) Sex-dependent regulation of social reward by oxytocin: an inverted U hypothesis. Neuropsychopharmacol 44: 97–110. DOI: 10.1038/s41386-018-0129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CA, Cardoso C, Ellenbogen MA. (2016) A meta-analytic review of the correlation between peripheral oxytocin and cortisol concentrations. Front Neuroendocrinol 43: 19–27. DOI: 10.1016/j.yfrne.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Brysbaert M, Stevens M. (2018) Power analysis and effect size in mixed effects models: A tutorial. J Cognit 1: 1–20. DOI: 10.5334/joc.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciotti-Saija C, Quintana DS, Alvares GA, et al. (2018) Reduced heart rate variability in a treatment-seeking early psychosis sample. Psychiatry Res 269: 293–300. DOI: 10.1016/j.psychres.2018.08.068. [DOI] [PubMed] [Google Scholar]
- Caffier PP, Erdmann U, Ullsperger P. (2003) Experimental evaluation of eye-blink parameters as a drowsiness measure. Eur J Appl Physiol 89: 319–325. DOI: 10.1007/s00421-003-0807-5. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. (1992) The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. JAMA J Am Med Assoc 267: 1244–1252. DOI: 10.1001/jama.1992.03480090092034. [DOI] [PubMed] [Google Scholar]
- Cosme G, Rosa PJ, Lima CF, et al. (2021) Pupil dilation reflects the authenticity of received nonverbal vocalizations. Sci Rep 11: 3733. DOI: 10.1038/s41598-021-83070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosme G, Tavares V, Nobre G, et al. (2022) Cultural differences in vocal emotion recognition: a behavioural and skin conductance study in Portugal and Guinea-Bissau. Psychol Res 86: 597–616. DOI: 10.1007/s00426-021-01498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker-Hopfe H, Kraemer S, Dorn H, et al. (2001) Time-of-day variations in different measures of sleepiness (MSLT, pupillography, and SSS) and their interrelations. Psychophysiol 38: 828–835. DOI: 10.1111/1469-8986.3850828. [DOI] [PubMed] [Google Scholar]
- Dodhia S, Hosanagar A, Fitzgerald DA, et al. (2014) Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacol 39: 2061–2069. DOI: 10.1038/npp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donets N, Dreyer J, Vallat R, et al. (2018) pyEntropy. [Google Scholar]
- Dreu CKWD. (2012) Oxytocin modulates cooperation within and competition between groups: An integrative review and research agenda. Horm Behav 61: 419–428. DOI: 10.1016/j.yhbeh.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Erdozain AM, Peñagarikano O. (2020) Oxytocin as treatment for social cognition, not there yet. Front Psychiatry 10: 930. DOI: 10.3389/fpsyt.2019.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SL, Monte OD, Noble P, et al. (2014) Intranasal oxytocin effects on social cognition: A critique. Brain Res 1580: 69–77. DOI: 10.1016/J.BRAINRES.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M, Büchel C. (2012) Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinol 37: 87–93. DOI: 10.1016/j.psyneuen.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Gutkowska J, Jankowski M, Antunes-Rodrigues J. (2014) The role of oxytocin in cardiovascular regulation. Braz J Med Biol Res 47: 206–214. DOI: 10.1590/1414-431X20133309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari-Dahan O, Bernstein A. (2014) A general approach-avoidance hypothesis of Oxytocin: Accounting for social and non-social effects of oxytocin. Neurosci Biobehav Rev 47: 506–519. DOI: 10.1016/j.neubiorev.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Hayes TR, Petrov AA. (2016) Mapping and correcting the influence of gaze position on pupil size measurements. Behav Res Methods 48: 510–527. DOI: 10.3758/s13428-015-0588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman R, Henik A, Cohen N. (2018) A novel blink detection method based on pupillometry noise. Behav Res Methods 50: 107–114. DOI: 10.3758/s13428-017-1008-1. [DOI] [PubMed] [Google Scholar]
- Howell DC. (2009) Multiple comparisons among treatment means. In: Potter J (ed) Statistics Methods for Psychology. 7th edn. Belmont, CA: Wadsworth Cengage Learning, pp. 366–367. [Google Scholar]
- Huang Y, Huang X, Ebstein RP, et al. (2021) Intranasal oxytocin in the treatment of autism spectrum disorders: A multilevel meta-analysis. Neurosci Biobehav Rev 122: 18–27. DOI: 10.1016/j.neubiorev.2020.12.028. [DOI] [PubMed] [Google Scholar]
- Jáuregui OI, Costanzo EY, de Achával D, et al. (2011) Autonomic nervous system activation during social cognition tasks in patients with schizophrenia and their unaffected relatives. Cognit Behav Neurol 24: 194. DOI: 10.1097/WNN.0b013e31824007e9. [DOI] [PubMed] [Google Scholar]
- Jawad MY, Ahmad B, Hashmi AM. (2021) Role of oxytocin in the pathogenesis and modulation of borderline personality disorder: A review. Cureus 13: e13190. DOI: 10.7759/cureus.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Kuhnert R-L, et al. (2012) Oxytocin increases heart rate variability in humans at rest: Implications for social approach-related motivation and capacity for social engagement. PLoS One 7: e44014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohova J, Czippelova B, Turianikova Z, et al. (2017) Preejection period as a sympathetic activity index: A role of confounding factors. Physiol Res 66: S265–S275. DOI: 10.33549/physiolres.933682. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Mendes WB, Appleton AA, et al. (2012) A heartfelt response: Oxytocin effects on response to social stress in men and women. Biol Psychol 90: 1–9. DOI: 10.1016/j.biopsycho.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster K, Goldbeck L, Puglia MH, et al. (2018) DNA methylation of OXTR is associated with parasympathetic nervous system activity and amygdala morphology. Soc Cognit Affective Neurosci 13: 1155–1162. DOI: 10.1093/scan/nsy086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Wessberg J, Ellingsen DM, et al. (2013) Oxytocin enhances pupil dilation and sensitivity to ‘hidden’ emotional expressions. Soc Cognit Affective Neurosci 8: 741–749. DOI: 10.1093/scan/nss062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipponen JA, Tarvainen MP. (2019) A robust algorithm for heart rate variability time series artefact correction using novel beat classification. J Med Eng Technol 43: 173–181. DOI: 10.1080/03091902.2019.1640306. [DOI] [PubMed] [Google Scholar]
- Lischke A, Lemke D, Neubert J, et al. (2017) Inter-individual differences in heart rate variability are associated with inter-individual differences in mind-reading. Sci Rep 7: 1–6. DOI: 10.1038/s41598-017-11290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tao H, Yang X, et al. (2019) Decreased serum oxytocin and increased homocysteine in first-episode schizophrenia patients. Front Psychiatry 10: 217. DOI: 10.3389/fpsyt.2019.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lory C, Kadlaskar G, McNally Keehn R, et al. (2020) Brief report: Reduced heart rate variability in children with autism spectrum disorder. J Autism Dev Disord 11: 4183–4190. DOI: 10.1007/S10803-020-04458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdtke H, Wilhelm B, Adler M, et al. (1998) Mathematical procedures in data recording and processing of pupillary fatigue waves. Vision Res 38: 2889–2896. DOI: 10.1016/S0042-6989(98)00081-9. [DOI] [PubMed] [Google Scholar]
- Mahmoud RA, Kaufmann T, Djupesland PG, et al. (2018) Low-dose intranasal oxytocin delivered with Breath Powered device modulates pupil diameter and amygdala activity: A randomized controlled pupillometry and fMRI study. Neuropsychopharmacol 44: 306–313. DOI: 10.1038/s41386-018-0241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Mucci F, et al. (2019) Sex-related differences in plasma oxytocin levels in humans. Clin Pract Epidemiol Mental Health 15: 58–63. DOI: 10.2174/1745017901915010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins D, Gabay AS, Mehta M, et al. (2020) Salivary and plasmatic oxytocin are not reliable trait markers of the physiology of the oxytocin system in humans. eLife 9: 1–19. DOI: 10.7554/ELIFE.62456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Thayer JF. (2018) How heart rate variability affects emotion regulation brain networks. Curr Opin Behav Sci 19: 98–104. DOI: 10.1016/J.COBEHA.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathôt S. (2018) Pupillometry: Psychology, physiology, and function. J Cognit 1: 16. DOI: 10.5334/joc.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meteyard L, Davies RAI. (2020) Best practice guidance for linear mixed-effects models in psychological science. J Mem Lang 112: 104092. DOI: 10.1016/j.jml.2020.104092. [DOI] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, et al. (2011) Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 12: 524–538. DOI: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mitchell MD, Haynes PJ, Anderson ABM, et al. (1981) Plasma oxytocin concentrations during the menstrual cycle. Eur J Obstet Gynecol Reprod Biol 12: 195–200. DOI: 10.1016/0028-2243(81)90077-0. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, et al. (2011) Oxytocin increases autonomic cardiac control: Moderation by loneliness. Biol Psychol 86: 174–180. DOI: 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Park G, Thayer JF. (2014) From the heart to the mind: cardiac vagal tone modulates top-down and bottom-up visual perception and attention to emotional stimuli. Front Psychol 5: 278. DOI: 10.3389/FPSYG.2014.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn K, Kazzer P, Lischke A, et al. (2013) Effects of intranasal oxytocin on pupil dilation indicate increased salience of socioaffective stimuli. Psychophysiol 50: 528–537. DOI: 10.1111/psyp.12042. [DOI] [PubMed] [Google Scholar]
- Quintana D, Kemp A, Alvares G, et al. (2013) A role for autonomic cardiac control in the effects of oxytocin on social behavior and psychiatric illness. Front Neurosci 7: 48. DOI: 10.3389/fnins.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ. (2020) An allostatic theory of oxytocin. Trends Cognit Sci 24: 515–528. DOI: 10.1016/j.tics.2020.03.008. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ, Outhred T, et al. (2012) Heart rate variability is associated with emotion recognition: Direct evidence for a relationship between the autonomic nervous system and social cognition. Int J Psychophysiol 86: 168–172. DOI: 10.1016/j.ijpsycho.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Lischke A, Grace S, et al. (2021) Advances in the field of intranasal oxytocin research: lessons learned and future directions for clinical research. Mol Psychiatry 26: 80–91. DOI: 10.1038/s41380-020-00864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2014) R: A Language and Environment for Statistical Computing. Vienna, Austria. [Google Scholar]
- Richman JS, Moorman JR. (2000) Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 278: H2039–49. DOI: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, et al. (2012) Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinol 37: 447–461. DOI: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. (1990) No adjustments are needed for multiple comparisons. Epidemiol 1: 43–46. DOI: 10.1097/00001648-199001000-00010. [DOI] [PubMed] [Google Scholar]
- Sabe M, Zhao N, Crippa A, et al. (2021) Intranasal oxytocin for negative symptoms of schizophrenia: Systematic review, meta-analysis, and dose-response meta-Analysis of randomized controlled trials. Int J Neuropsychopharmacol 24: 601–614. DOI: 10.1093/ijnp/pyab020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonia A, Nappi RE, Pontillo M, et al. (2005) Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav 47: 164–169. DOI: 10.1016/J.YHBEH.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Saville DJ. (1990) Multiple comparison procedures: The practical solution. Am Stat 44: 174. DOI: 10.2307/2684163. [DOI] [Google Scholar]
- Schleicher R, Galley N, Briest S, et al. (2008) Blinks and saccades as indicators of fatigue in sleepiness warnings: Looking tired? Ergon 51: 982–1010. DOI: 10.1080/00140130701817062. [DOI] [PubMed] [Google Scholar]
- Schoormans D, Kop WJ, Kunst LE, et al. (2020) Oxytocin effects on resting-state heart rate variability in women: The role of childhood rearing experiences. Compr Psychoneuroendocrinol 3: 100007. DOI: 10.1016/j.cpnec.2020.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann A, Andrack C, Bär KJ. (2017) Differences of sympathetic and parasympathetic modulation in major depression. Prog Neuro-Psychopharmacol Biol Psychiatry 79: 324–331. DOI: 10.1016/j.pnpbp.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Schumann A, Kietzer S, Ebel J, et al. (2020) Sympathetic and parasympathetic modulation of pupillary unrest. Front Neurosci 14: 178. DOI: 10.3389/fnins.2020.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer F, Ginsberg JP. (2017) An overview of heart rate variability metrics and norms. Front Public Health 5: 258. Available at: https://www.frontiersin.org/articles/10.3389/fpubh.2017.00258 (accessed 6 December 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A. (2016) The social salience hypothesis of oxytocin. Biol Psychiatry 79: 194–202. DOI: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Feifel D. (2016) Potential of oxytocin in the treatment of schizophrenia. CNS Drugs 30: 193–208. DOI: 10.1007/s40263-016-0315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock S, Bremme K, Uvnäs-moberg K. (1991) Plasma levels of oxytocin during the menstrual cycle, pregnancy and following treatment with HMG. Hum Reprod 6: 1056–1062. DOI: 10.1093/OXFORDJOURNALS.HUMREP.A137484. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen JP, Lipponen JA, et al. (2014) Kubios HRV – Heart rate variability analysis software. Comput Methods Programs Biomed 113: 210–220. DOI: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Tracy LM, Gibson SJ, Labuschagne I, et al. (2018) Intranasal oxytocin reduces heart rate variability during a mental arithmetic task: A randomised, double-blind, placebo-controlled cross-over study. Prog Neuro-Psychopharmacol Biol Psychiatry 81: 408–415. DOI: 10.1016/j.pnpbp.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Tulppo MP, Antti KM, Arto HJ, et al. (2005) Physiological background of the loss of fractal heart rate dynamics. Circulation 112: 314–319. DOI: 10.1161/CIRCULATIONAHA.104.523712. [DOI] [PubMed] [Google Scholar]
- Wang Y, Toor SS, Gautam R, et al. (2011) Blink frequency and duration during perimetry and their relationship to test-retest threshold variability. Invest Ophthalmol Visual Sci 52: 4546–4550. DOI: 10.1167/iovs.10-6553. [DOI] [PubMed] [Google Scholar]
- Winterton A, Westlye LT, Steen NE, et al. (2021) Improving the precision of intranasal oxytocin research. Nat Hum Behav 5: 9–18. DOI: 10.1038/s41562-020-00996-4. [DOI] [PubMed] [Google Scholar]
- Yang T, Simmons A, Matthews S, et al. (2007) Increased amygdala activation is related to heart rate during emotion processing in adolescent subjects. Neurosci Lett 428: 109–114. DOI: 10.1016/j.neulet.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenina M, Kosilo M, da Cruz J, et al. (2022) Temporal dynamics of intranasal oxytocin in human brain electrophysiology. Cereb Cortex 32: 3110–3126. DOI: 10.1093/cercor/bhab404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Zhang H, Wang P, et al. (2022) Oxytocin and serotonin in the modulation of neural function: Neurobiological underpinnings of autism-related behavior. Front Neurosci 16: 1143. DOI: 10.3389/fnins.2022.919890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jop-10.1177_02698811231158233 for Temporal profile of intranasal oxytocin in the human autonomic nervous system at rest: An electrocardiography and pupillometry study by Gonçalo Cosme, Patrícia Arriaga, Pedro J. Rosa, Mitul A. Mehta and Diana Prata in Journal of Psychopharmacology