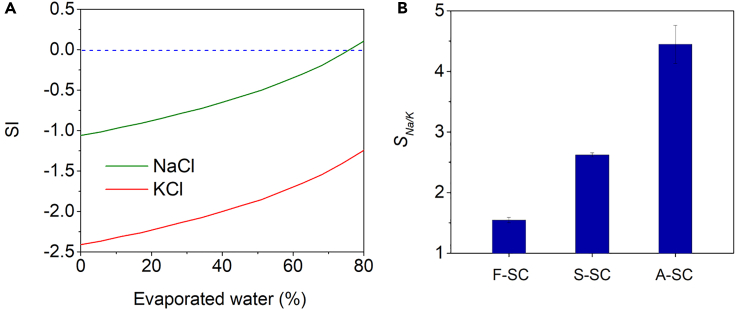
Figure 3.
Ion-selective crystallization facilitated by SCs
(A) Simulated changes in saturation index (SI) of NaCl (green) and KCl (red) in a solution containing 10 wt % NaCl and 2 wt % KCl because of evaporation. The dotted line represents the solubility equilibrium, indicating the point at which the solution is saturated. Below the line, re-dissolution is preferred rather than crystallization.
(B) The selectivity of Na+ to K+ in the crystallized salt produced from the solution with F-SC, S-SC, and A-SC after 12 h of solar crystallization. Data are represented as mean ± SD.