Abstract
Background:
The clinical significance of Epstein-Barr virus (EBV) and human papillomavirus (HPV) infection in nasopharyngeal carcinoma (NPC) is unclear.
Methods:
Three hundred and forty three patients with NPC diagnosed between 1998 and 2017 and treated at our institution were included. Chi-square was used to identify characteristics associated with viral status. Kaplan-Meier methods were used to estimate overall survival (OS) and Cox proportional regression was used to identify prognostic factors.
Results:
Patients with HPV-associated NPC were more likely to have a positive smoking history and to present at a higher T classification. At a median follow-up time of 59.9 months (range: 0.1–222.4 months), there were no differences in OS (P = .198), time to local failure (LF, P = .403), or time to distant metastasis (DM, P = .849) between the viral subgroups. Older age (hazard ratio [HR]: 2.242, 95% confidence interval [CI] 1.374–3.659, P = .001) and higher overall stage (HR: 2.047, 95% CI 1.235–3.391, P = .005) were prognostic for worse OS.
Conclusion:
In our population, viral status was not prognostic for OS, LF, or DM.
Keywords: EBV, Epstein-Bar virus, HPV, human papillomavirus, nasopharyngeal carcinoma
1 |. INTRODUCTION
Nasopharyngeal carcinoma (NPC) arises from the nasopharynx epithelium and can invade locally, spread to nearby lymph nodes, or metastasize to distant organs. Globally there are approximately 80 000 new diagnoses of NPC per year, representing 0.6% of all cancers.1 NPC is rare in North America with an incidence of 0.3 to 0.7/100 000 and is more common in certain geographic regions (Southeast Asia, North and East Africa) and among particular ethnicities (Intuits, Aleuts, Asians).2 NPC has a multifactorial etiology in which genetic factors and environmental factors can influence susceptibility.3 Smoking, alcohol, and a high intake of salt-preserved food have been found to increase risk, especially in endemic areas such as Southeast Asia.4,5 Viral infections, in particular Epstein-Barr virus (EBV) and human papillomavirus (HPV), are also etiological agents for the development of NPC.6
NPC is categorized based on histology into keratinizing squamous cell carcinoma (previously WHO type I), differentiated non-keratinizing carcinoma (previously WHO type II), poorly differentiated or undifferentiated non-keratinizing carcinoma (previously WHO type III), and basaloid squamous cell carcinoma.7 EBV is associated with endemic forms of NPC that are prevalent in regions such as southeast China and is typically characterized as poorly differentiated non-keratinizing. HPV is associated with nonendemic forms of NPC, has a lower incidence, and is enriched among Caucasians.1 HPV infection in NPC ranges from 9% to 52.9%, depending on the ethnicity and geographic distribution of the population under investigation as well as viral detection methods.8–13 Although a few earlier studies suggested that co-infection could occur in NPC,11,14 further analysis has shown that EBV and HPV infections are mutually exclusive.15–18 Finally, viral negative NPC, in which there is neither EBV nor HPV infection, has also been identified and is primarily found in nonendemic populations.
The rise in prevalence of HPV has led to a shift in the epidemiology and clinical management of head and neck squamous cell carcinomas. This is most established for oropharyngeal cancer (OPC) in which 90% of tumors are positive for high-risk HPV.11 Furthermore, HPV-positive OPC has a striking 28% to 58% reduced risk of death compared to HPV-negative OPC. For NPC, current guidelines recommend HPV testing in EBV-negative NPC; however, it is unclear whether HPV status of NPC tumors has prognostic significance and should be used to guide management. Here, we have collected the largest single institution database of patients with EBV-positive, HPV-positive, and viral negative NPC to address these questions by investigating risk factors, disease characteristics, and treatment responses for NPC at our institution.
2 |. MATERIALS AND METHODS
2.1 |. Patient selection
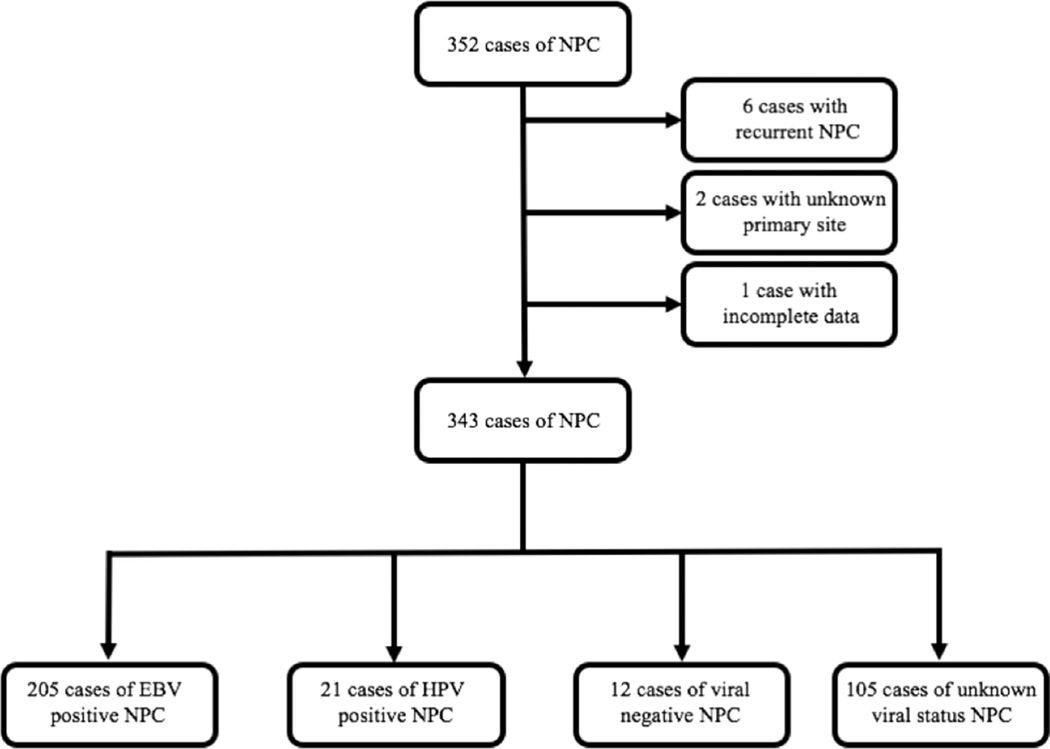
As part of an institutional review board-approved study, we retrospectively reviewed the records of all consecutive patients who had a diagnosis of NPC between 1998 and 2017 at Memorial Sloan-Kettering Cancer Center and identified 352 patients. Of these, six patients with a diagnosis of recurrent NPC, two patients with a diagnosis of squamous cell carcinoma of unknown primary and one patient whose information was restricted due to privacy was excluded. Among the remaining 343 patients, 169 tested positive for EBV by in situ hybridization (ISH) for EBV-encoded RNA. Twenty-one were HPV-positive by p16 immunohistochemistry (IHC) or by HPV PCR-Mass-Array, and 12 were negative for both EBV and HPV by these methods. We also included 36 patients without EBV pathological results but who were of East Asian ethnicity and had NPC with non-keratinizing histology in our EBVpositive category because previous studies have shown that almost all East Asians with non-keratinizing NPC had EBV-positive NPC.15,19–21 Within our own sample, among Asian patients with known EBV status and nonkeratinizing histology, 72 out of 74 (97%) were EBV-positive, thereby affirming our assumption. In total, of the 238 patients with known viral status, 205 patients were characterized as EBV-positive, 21 were HPV-positive, and 12 were viral negative. Hundred and five patients (diagnosed between April 27, 1998 to September 23, 2015, 92 of whom were diagnosed before 2010) were characterized as having unknown viral background, 68 were white, 15 were black, 9 were Hispanic, and 13 were of other or unknown ethnicity. Figure 1 shows selection and categorization of patients into all viral subgroups.
FIGURE 1.
Schema for the selection and categorization of nasopharyngeal carcinoma cases
2.2 |. EBV and HPV detection
EBV was detected using ISH for EBV-encoded RNA on formalin-fixed, paraffin-embedded tissue sections using the EBER 1 DNP probe (Roche, Basel, Switzerland) according to the manufacturer’s protocol. A positive hybridization was based on intensity of nuclear signal in tumor cells.
HPV status was assessed by IHC of p16, which is an established biomarker for HPV oncoprotein function in OPCs22 and by HPV PCR-MassArray. IHC for p16 was performed on formalin-fixed, paraffin-embedded tissue using p16 antibody clone E6H4 (Roche, Basel, Switzerland) according to the manufacturer’s protocol. Samples with strong and diffuse staining in greater than 70% of tumor cells were classified as HPV-positive. HPV PCR-MassArray was performed as previously described.23
2.3 |. Statistical analyses
Data were analyzed using SPSS, version 25 (IBM). For the total 343 patients Pearson Chi-square test was used to assess for any association between patient, tumor and treatment characteristics, and viral status (EBV-positive, HPV-positive, viral negative or unknown) and Kaplan-Meier method was used for analysis of overall survival (OS), local failure (LF), regional failure (RF), local or regional failure (LRF), progression-free survival (PFS), and distant metastasis (DM). Univariate Cox-proportional hazard analysis was used to evaluate patient, tumor, and treatment characteristics as prognostic factors for OS. The following characteristics were included in univariate analysis: age at diagnosis (<60 or ≥60), gender (male or female), race (white, Asian or other), Karnofsky Performance Status (KPS, <90 or ≥90), smoking history (<20 pack years or ≥20 pack years), histology (nonkeratinizing or other), tumor classification (T1 or T2-T4), nodal involvement (N0 or N1-N3), overall stage (I-III or IVAIVC), chemotherapy received (none, concurrent and adjuvant, concurrent, concurrent and neoadjuvant), time to start radiotherapy (RT) (<2 months or ≥2 months), and viral status (EBV-positive, HPV-positive, viral negative, or unknown). For multivariate analysis age, gender, KPS, smoking history, overall stage, chemotherapy received, time to start RT, and viral status were included. For all analysis, hypothesis testing was two-sided with a 5% level of significance.
3 |. RESULTS
Our final cohort included 343 eligible patients with a median age of 52 years (range 10–92 years). The median follow-up time for surviving patients from the date of diagnosis was 59.9 months (range: 0.1–222.4 months). Two hundred and forty seven patients were male (72.0%) and 96 patients were female (28.0%). Two hundred and five patients were EBV-positive (59.8%), 21 patients were HPV-positive (6.1%), 12 patients were viral negative (3.5%), and 105 were of unknown viral status (30.6%). Baseline demographic and tumor characteristics by EBV and HPV status are presented in Table 1. Pearson Chi-square test was used to assess for any association between patient, tumor and treatment characteristics, and viral status (EBV-positive, HPV-positive, viral negative and unknown). HPV-positive tumors were associated with higher tumor classification (P = .044) than EBV-associated tumors. HPV-positive patients were also more likely to have a positive smoking history (P < .001). There was no significant association between patient age, nodal involvement or overall stage, and viral status of the tumor.
TABLE 1.
Patient, tumor and treatment characteristics by viral status (N = 343)
| Characteristic | Total N (%) | EBV positive (N = 205) N (%) | HPV Positive (N = 21) N (%) | Viral negative (N = 12) N (%) | Unknown (N = 105) N (%) | Pearson χ2 P |
|---|---|---|---|---|---|---|
| Age | ||||||
| <60 | 241 (70.3) | 154 (75.1) | 12 (57.1) | 9 (75.0) | 66 (62.9) | .074 |
| ≥60 | 102 (29.7) | 51 (24.9) | 9 (42.9) | 3 (25.0) | 39 (37.1) | |
| Gender | ||||||
| Male | 247 (72.0) | 149 (72.7) | 17 (81.0) | 9 (75.0) | 72 (68.6) | .671 |
| Female | 96 (28.0) | 56 (27.3) | 4 (19.0) | 3 (25.0) | 33 (31.4) | |
| Race | ||||||
| White | 155 (46.7) | 65 (33.2) | 15 (71.4) | 7 (63.6) | 68 (65.4) | <.0001 |
| Asian | 123 (37.0) | 111 (56.6) | 1 (4.8) | 3 (27.3) | 8 (7.7) | |
| Other | 54 (16.3) | 20 (10.2) | 5 (23.8) | 1 (9.1) | 28 (26.9) | |
| KPS | ||||||
| <90 | 63 (19.0) | 35 (17.5) | 5 (23.8) | 2 (20.0) | 21 (21.0) | .832 |
| ≥90 | 268 (81.0) | 165 (82.5) | 16 (76.2) | 8 (80.0) | 79 (79.0) | |
| Smoking history | ||||||
| <20 | 252 (75.7) | 169 (85.3) | 11 (52.4) | 6 (54.5) | 66 (64.1) | <.0001 |
| ≥20 | 81 (24.3) | 29 (14.7) | 10 (47.6) | 5 (45.5) | 37 (35.9) | |
| Histology | ||||||
| Nonkeratinizing | 220 (88.0) | 187 (91.2) | 15 (71.4) | 9 (75.0) | 9 (75.0) | <.0001 |
| Other | 30 (12.0) | 18 (8.8) | 6 (28.6) | 3 (25.0) | 3 (25.0) | |
| Tumor classification | ||||||
| T1 | 116 (34.1) | 78 (38.6) | 2 (9.5) | 4 (33.3) | 32 (30.5) | .044 |
| T2-T4 | 224 (65.9) | 124 (61.4) | 19 (90.5) | 8 (66.7) | 73 (69.5) | |
| Nodal involvement | ||||||
| N0 | 70 (20.6) | 34 (16.8) | 5 (23.8) | 1 (8.3) | 30 (28.6) | .070 |
| N1–3 | 270 (79.4) | 168 (83.2) | 16 (76.2) | 11 (91.7) | 75 (71.4) | |
| Overall stage group | ||||||
| I-III | 233 (68.1) | 139 (68.1) | 11 (52.4) | 9 (75.0) | 74 (70.5) | .403 |
| IVA-IVC | 109 (31.9) | 65 (31.9) | 10 (47.6) | 3 (25.0) | 31 (29.5) | |
| Chemotherapy | ||||||
| None | 27 (7.9) | 16 (7.8) | 1 (4.8) | 0 (0) | 10 (9.6) | .012 |
| Concurrent and adjuvant | 194 (56.9) | 109 (53.4) | 8 (38.1) | 8 (66.7) | 69 (66.3) | |
| Concurrent alone | 76 (22.3) | 45 (22.1) | 11 (52.4) | 3 (25.0) | 17 (16.3) | |
| Concurrent and neoadjuvant | 44 (12.9) | 34 (16.7) | 1 (4.7) | 1 (8.3) | 8 (7.7) | |
| Time to start radiotherapy | ||||||
| <2 months | 162 (47.2) | 82 (40.0) | 9 (75.0) | 7 (58.3) | 64 (61.0) | .005 |
| ≥2 months | 181 (52.8) | 123 (60.0) | 12 (25.0) | 5 (41.7) | 41 (39.0) |
Note: For a few patients, data for certain characteristics was not available. These patients were excluded from the respective Chi-square analysis.
Abbreviations: EBV, Epstein-Barr virus; HPV, human papillomavirus.
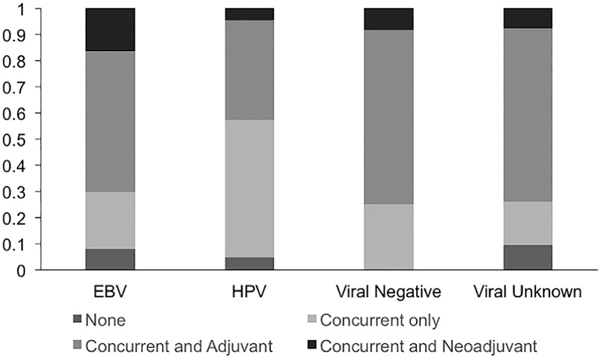
Among the 339 cases treated with definitive intensity-modulated radiotherapy (IMRT), 322 were treated to a 70 Gray (Gy) dose. Fifteen cases (10 EBV, 1 HPV, 1 viral negative and 3 unknown) were treated to less than 70 Gy (range: 36–64 Gy) due to significant toxicities, and 2 (1 HPV and 1 unknown) were treated with more than 70 Gy, due to treatment interruptions. Four cases (3 EBV and 1 HPV) were treated with definitive protons. In addition, 2 HPV-positive NPC patients were given a proton boost following IMRT. The chemotherapy regimens for EBV-positive, HPV-positive, viral negative, and viral unknown patients are shown in Figure 2.
FIGURE 2.
Chemotherapy regimens used for different viral subgroups
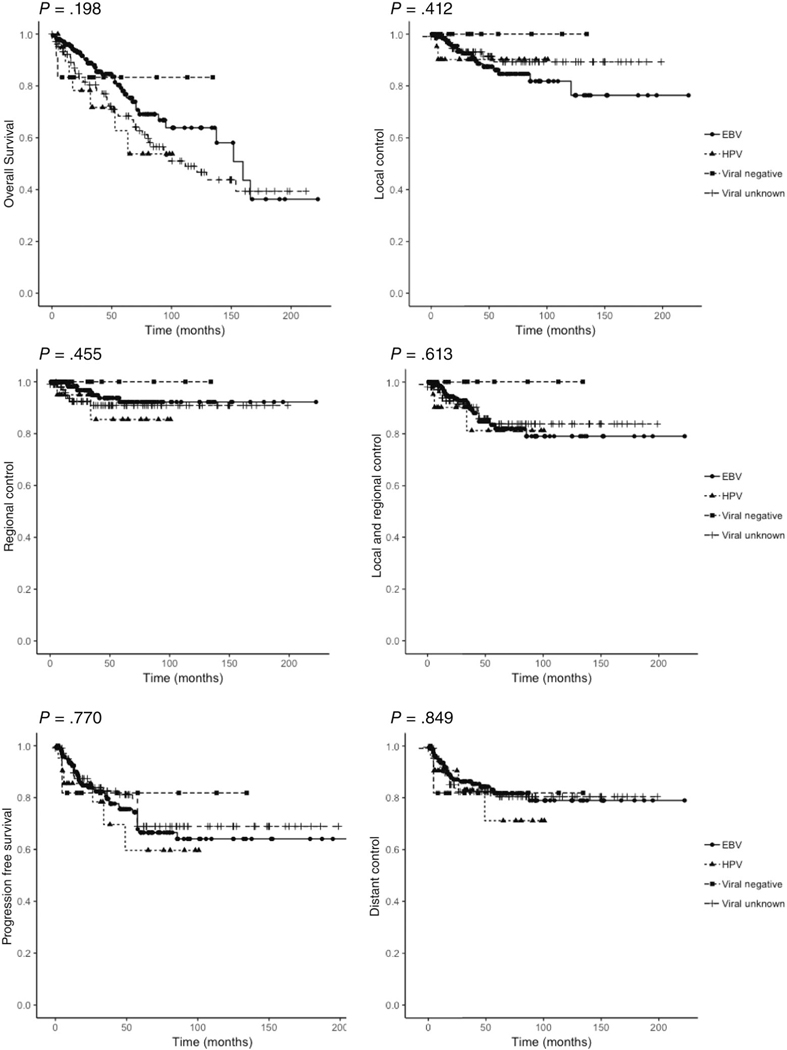
EBV-positive, HPV-positive, viral negative, and viral unknown NPC patients showed no significant difference in OS (P = .198), LF (P = .412), RF (P = .455), LRF (P = .613), PFS (P = .770), or DM (P = .849), as shown in Figure 3. Among the 42 deaths within the EBV-positive subgroup, 13 had local failure, 4 had regional failure, and 23 had distant metastasis at time of death. For the HPV-positive subgroup, there were 7 deaths, 2 with local failure, 1 with regional failure, and 4 with distant metastasis. The viral negative group had 2 deaths, one of which showed distant metastasis, and neither of which had local or regional failure.
FIGURE 3.
The Kaplan-Meier curves for viral subgroups are shown in different colors (blue: EBV, red: HPV, green: viral negative, orange: viral unknown). P-values from log rank analysis are shown
On univariate Cox regression analysis older age, lower KPS, greater smoking history, histology other than non-keratinizing, greater tumor classification, and greater overall stage were associated with poorer OS. Viral status was not prognostic for OS on univariate analysis. On multivariate Cox regression analysis older age (hazard ratio [HR] 2.242; 95% confidence interval [CI] 1.374–3.659, P = .001), and greater overall stage (HR 2.047; 95% CI 1.235–3.391, P = .005) were associated with poorer OS. Higher KPS (HR 0.485; 95% CI 0.292–0.805, P = .005) was associated with improved OS (Table 2).
TABLE 2.
Associations between baseline characteristics and overall survival (OS) (N = 343)
| OS univariate analysis |
OS multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | P | HR | 95% CI | P |
| Age | ||||||
| <60 | REF | REF | ||||
| ≥60 | 2.857 | 1.907–4.281 | <.0001 | 2.242 | 1.374–3.659 | .001 |
| Gender | ||||||
| Male | REF | REF | ||||
| Female | 0.604 | 0.368–0.991 | .046 | 0.828 | 0.475–1.445 | .507 |
| Race | ||||||
| White | REF | |||||
| Asian | 0.784 | 0.493–1.246 | .303 | |||
| Other | 1.259 | 0.722–2.194 | .417 | |||
| KPS | ||||||
| <90 | REF | REF | ||||
| ≥90 | 0.344 | 0.223–0.529 | <.0001 | 0.485 | 0.292–0.805 | .005 |
| Smoking history | ||||||
| <20 | REF | REF | ||||
| ≥20 | 1.875 | 1.224–2.873 | .004 | 1.518 | 0.913–2.521 | .107 |
| Histology | ||||||
| Nonkeratinizing | REF | |||||
| Other | 1.975 | 1.282–3.043 | .002 | |||
| Tumor classification | ||||||
| T1 | REF | |||||
| T2-T4 | 2.050 | 1.239–3.393 | .005 | |||
| Nodal involvement | ||||||
| N0 | REF | |||||
| N1-N3 | 0.955 | 0.577–1.581 | .858 | |||
| Overall stage |
||||||
| I-III | REF | REF | ||||
| IVA-IVC | 2.638 | 1.760-3.954 | <.0001 | 2.047 1.235 | 3.391 | .005 |
| Chemotherapy | ||||||
| None | REF | REF | ||||
| Concurrent and adjuvant | 1.464 | 0.529–4.056 | .463 | 0.967 | 0.330–2.838 | .952 |
| Concurrent alone | 2.479 | 0.847–7.250 | .097 | 1.252 | 0.400–3.916 | .699 |
| Concurrent and neoadjuvant | 4.385 | 1.476–13.03 | .008 | 2.228 | 0.673–7.378 | .190 |
| Time to start RT | ||||||
| <2 months | REF | REF | ||||
| ≥2 months | 1.088 | 0.727–1.628 | .683 | 1.106 | 0.660–1.851 | .702 |
| Viral status | ||||||
| EBV positive | REF | REF | ||||
| HPV positive | 1.799 | 0.807–4.011 | .151 | 1.227 | 0.508–2.960 | .649 |
| Viral negative | 0.897 | 0.217–3.708 | .880 | 1.461 | 0.337–6.327 | .612 |
| Unknown | 1.494 | 0.974–2.292 | .066 | 1.116 | 0.667–1.870 | .676 |
Abbreviations: CI, confidence interval; EBV, Epstein-Barr virus; HR, hazard ratio; HPV, human papillomavirus; OS, overall survival.
4 |. DISCUSSION
Here we present the largest single-institution evaluation of patients with EBV-positive, HPV-positive, and viral negative NPC (Table 3). A number of retrospective studies have compared OS between HPV-positive and HPV-negative NPC24–28 or between EBV-positive and EBV-negative NPC29–32; however, as these studies did not identify and directly compare both EBV-positive and HPV-positive cases, it is difficult to interpret whether there is a survival advantage associated with a particular viral infection or for NPC cases that are not virally associated. Five previous studies have directly compared OS between EBV-positive, HPV-positive and viral negative NPC cases, and have come to different conclusions. Two studies15,18 found no differences in OS. The remaining three14,16,17 all found that viral negative NPC (HPV and EBV-negative) had worse OS than virally associated NPC. Stenmark et al found that both viral negative and HPV-positive NPC had worse OS than EBV-positive NPC, with viral negative tumors faring worse than HPV-positive tumors. HPV-positive and viral negative tumors showed poorer locoregional control (48.1% and 26.4%, respectively) than EBV-positive tumors (87.3%), which may have contributed to their worse overall survival. Wilson et al found improved locoregional control for virally associated NPC compared to viral negative NPC, although there was no difference between EBV-positive and HPV-positive tumors. The local control rates between the different viral subsets were not investigated in the other three studies.14,15,18
TABLE 3.
Previous studies evaluating the prognostic significance of viral status in nasopharyngeal carcinoma (NPC)
| Study | No. of Pts (EBV+/ HPV+/viral negative) | Study dates | Median follow-up time (Mo) | Comparison | Conclusion |
|---|---|---|---|---|---|
| Yip et al29 | 80 | 1985–1992 | 136.8 | EBV positive vs EBV negative | EBV positive had increased OS compared to EBV negative |
| Lin et al30 | 24 | 1993–2010 | 48 | EBV positive vs EBV negative | No significant difference in OS |
| Jiang et al31 | 86 | 2000–2014 | 36 | EBV positive vs EBV negative | EBV positive had increased OS, PFS and LRC compared to EBV negative |
| Peng et a32 | 1106 | 2009–2012 | 49.8 | EBV positive vs EBV negative | Among patients with advanced stage (III-IVB) NPC, EBV positive had decreased OS and PFS compared to EBV negative |
| Huang et al24 | 31 | 2003–2004 | 38 | HPV positive vs HPV negative | No significant difference in PFS |
| Atighechi et al25 | 41 | 1995–2007 | 48 | HPV positive vs HPV negative | HPV negative had higher recurrence and mortality rates compared to HPV positive |
| Fakhry et al26 | 125 | 1995–2012 | 42 | HPV positive vs HPV negative | No significant difference in OS |
| Li et al27 | NCDB: 1006 | 2010–2014 | HPV positive vs HPV negative | No significant difference in OS | |
| Verma et al28 | NCDB: 956 | 2004–2013 | 23 | HPV positive vs HPV negative | No significant difference in OS |
| Dogan et al14 | 63 (38/6/19) | 1981–2012 | 38 | EBV vs HPV vs viral negative | Viral negative had worse OS than HPV or EBV positive. No significant difference in OS between HPV and EBV positive |
| Robinson et al15 | 67(47/11/9) | 2000–2012 | 26 | EBV vs HPV vs viral negative | No significant difference in OS between groups |
| Wilson et al16 | 13 (5/4/4) | 2002–2013 | 30.1 | EBV vs HPV vs viral negative | Viral association (EBV or HPV) was a predictor of increased OS. No significant difference in OS between EBV and HPV |
| Stenmark et al17 | 62 (26/18/17) | 1985–2011 | 84 | EBV vs HPV vs viral negative | Viral negative had worst OS, PFS and LRC. HPV positive had worse OS, PFS, and LRC than EBV positive |
| Kano et al18 | 59 (49/2a/8) | 1996–2015 | 58 | EBV vs HPV vs viral negative | No significant difference in OS between groups |
Abbreviations: CI, confidence interval; EBV, Epstein-Barr virus; HR, hazard ratio; HPV, human papillomavirus; OS, overall survival.
Patients were EBV and HPV positive.
In our own study despite presenting with a higher tumor classification, patients with HPV-positive tumors did not show greater local or regional failure rates than EBV-positive NPC patients. Moreover, we found no significant difference in OS between EBV-positive, HPV-positive, and viral negative NPC. The lack of consensus between different studies on the prognostic importance of viral association in NPC is likely due to a number of factors. This could include the small number of patients in the studies published to date, ethnic and geographic variations in study populations, and variability in follow-up times. The three previous studies that found no difference in OS between viral subgroups of NPC had only 2, 4, or 6 HPV-positive patients.14,16,18 Stenmark et al focused on a non-endemic Midwest population, included 18 HPV-positive patients in their dataset and observed a difference in OS between viral subgroups. We included 21 HPV-positive patients within our analysis. Our patient population coming from New York City is quite different from the Midwest population used in the Stenmark study and variations between these populations almost certainly contributed to the differences in clinical outcomes that we observed.
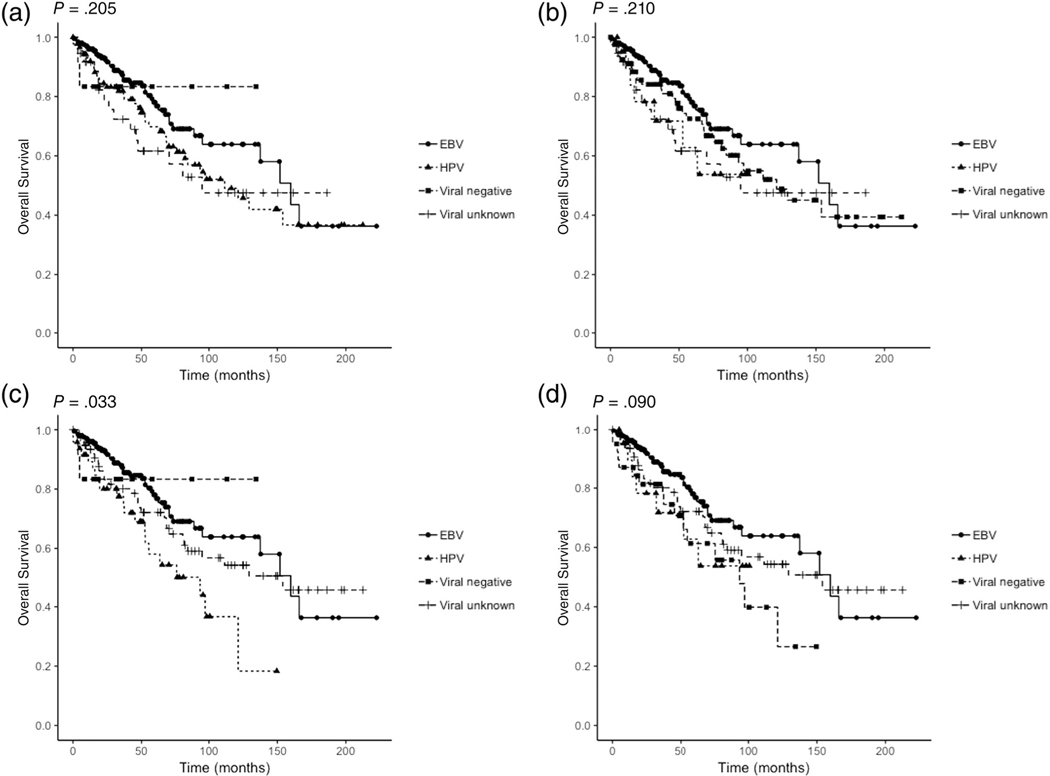
It is also possible, however, that with a greater number of patients within our HPV group, we could have detected significant clinical differences between the viral subgroups. We decided to explore this further by investigating whether reclassifying patients in the viral unknown group (105 patients) into either the HPV-positive or viral negative subgroups would produce different results (Table S1). Among these 105 patients of unknown viral background, the racial distribution was: 68 white, 15 black, 9 Hispanic, and 13 of other or unknown ethnicity. For histology: 59 cases were non-keratinizing and 46 had a histology other than non-keratinizing. There is some evidence that white patients, and NPC tumors with histology other than non-keratinizing have a greater likelihood of being HPV-positive or viral negative.15,33 We reclassified white patients in the unknown viral group (N = 68) into either the HPV-positive (Analysis 1) or viral negative (Analysis 2) subgroups. We also reclassified white patients with histology other than non-keratinizing in the unknown viral group (N = 27) into either the HPV-positive (Analysis 3) or viral negative (Analysis 4) subgroups. We repeated Kaplan-Meier overall survival analysis for each of these analyses (Figure 4). Interestingly, when white patients with histology other than non-keratinizing were reclassified as HPV-positive (which now had 48 patients instead of 21), there was a significant difference in overall survival between viral subgroups (P = .033). Furthermore, univariate Cox analysis showed that viral classification was predictive of overall survival (EBV vs HPV P = .004); however, the result was not significant on multivariate analysis. This result highlights that the limited number of patients in HPV-positive and viral negative subgroups found in the studies to date likely contributes to the variability in conclusions presented. Pooling participants from different institutions could lead to stronger conclusions by offsetting the limited number of patients and also possibly the variability between populations from different geographic regions.
FIGURE 4.
Kaplan-Meier overall survival curves for four additional analyses are shown. A, Analysis 1; B, Analysis 2; C, Analysis; 3 D, Analysis 4. P-values from log rank analysis are shown. Viral subgroups are shown by different colors (blue: EBV, red: HPV, green: viral negative, orange: viral unknown)
Another factor to consider is the length of follow-up time. Stage I and Stage II NPC have 10-year survival rates of 98% and 60%, respectively, whereas advanced stage NPC (stage III or IV) has a median survival of 3 years.34 Although Robinson et al had a sizeable number of HPV-positive NPC cases (11 cases) they did not observe a difference in OS between EBV-positive and HPV-positive viral groups. This could be due in part to the relatively short median follow-up time of 26 months, in comparison Stenmark et al had a median follow-up time of 84 months. In our own study, the overall median follow-up time was 59.9 months for the entire cohort; however, there were differences among the viral subgroups: median follow-up times for EBV-positive, HPV-positive, and viral negative patients were 47.9, 65.1, and 34.7 months, respectively. The limited follow-up time of the viral negative subgroup in particular may have prevented us from detecting more subtle differences in clinical outcomes.
A final source of variability between studies is the method used to identify HPV-positive NPC. Whereas EBV is almost uniformly detected using EBV ISH, currently used methods for HPV detection include p16 IHC, HPV PCR, and HPV ISH. p16 is an established biomarker for HPV oncoprotein function in OPCs22 and is consistently negative in EBV-positive NPC11; however, there is controversy over the correlation between positive p16 staining and HPV DNA detection.16,17 Although a two-tiered approach using p16 and HPV ISH has been advocated for NPC,16 there is also the risk that the low sensitivity of HPV ISH or PCR, due to DNA degradation or undetected types of HPV, may exclude true positive cases. In our dataset, 17 of the 21 p16 positive cases were verified by HPV PCR and in only one of the cases was HPV PCR undetected. As a result, we believe it is acceptable to identify HPV-positive cases using p16 alone, at least until consistent testing guidelines for accurate viral categorization are established.
Despite the dispute over the prognostic significance of viral status, a number of consistent findings have been observed. For instance, compared to EBV-positive tumors, HPV-associated NPC show increased propensity for local spread. Among our cohort, we found that HPV-positive NPC had greater local tumor burden than EBV-positive NPC. At diagnosis, 90.5% of HPV-positive NPC tumors were T2 to T4 compared to 61.4% of EBV-positive NPC tumors. We also observed that certain patient characteristics correlated with viral status in NPC. In studies of OPC, HPV-positive OPC patients were typically younger and less likely to use alcohol or tobacco.35,36 In contrast, HPV-positive NPC patients are more likely to have a positive smoking history.17,26,30 Likewise, in our study we found that HPV-positive patients had a significantly greater smoking history: 57.9% of HPV-positive patients were either a former or current smoker, compared to 35.3% of EBV-positive patients. Studies of cervical cancer have suggested that smoking can facilitate HPV infection37 and impact the initiation and progression of HPV-associated malignancy.38–41 Further investigation of this association can have significant implications for the development of screening guidelines and risk-stratified treatment paradigms.
This study represents a single-institution evaluation of EBV, HPV, and viral negative-associated NPC. The main limitations of this study include its retrospective nature and the limited number of patients in the HPV-positive and viral negative subgroups. In addition, a longer follow-up time, in particular for the viral negative group, may have revealed more subtle differences in clinical outcomes. Further investigations with larger or combined datasets are necessary to validate our results as well as provide an accurate assessment of the incidence and prevalence of HPV-associated NPC among different patient populations.
Supplementary Material
ACKNOWLEDGEMENTS
Funding support by NIH R01CA129182 and NIH/NCI Cancer Center Support Grant P30 CA008748.
Funding information
National Cancer Institute, Grant/Award Number: P30 CA008748; National Institutes of Health, Grant/Award Number: R01CA129182
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Chua M, Wee J, Hui E, Chan A. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–1024. [DOI] [PubMed] [Google Scholar]
- 2.Petersson F.Nasopharyngeal carcinoma: a review. Semin Diagn Pathol. 2015;32(1):54–73. [DOI] [PubMed] [Google Scholar]
- 3.Lu S, Day N, Degos L, et al. Linkage of a nasopharyngeal carcinoma susceptibility locus to the HLA region. Nature. 1990; 346(6283):470–471. [DOI] [PubMed] [Google Scholar]
- 4.Guo X, Johnson R, Deng H, et al. Evaluation of nonviral risk factors for nasopharyngeal carcinoma in a high-risk population of Southern China. Int J Cancer. 2009;124(12):2942–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu F, Xiong D, Xu Y, et al. An epidemiological and molecular study of the relationship between smoking, risk of nasopharyngeal carcinoma, and Epstein-Barr virus activation. J Natl Cancer Inst. 2012;104(18):1396–1410. [DOI] [PubMed] [Google Scholar]
- 6.Wei W, Sham J. Nasopharyngeal carcinoma. Lancet. 2005;365 (9476):2041–2054. [DOI] [PubMed] [Google Scholar]
- 7.Pathology Barnes L. and Genetics of Head and Neck Tumours. LyoFn, rance: IARC Press; 2005. [Google Scholar]
- 8.Rassekh C, Rady P, Arany I, et al. Combined Epstein-Barr virus and human papillomavirus infection in nasopharyngeal carcinoma. Laryngoscope. 1998;108:362–367. [DOI] [PubMed] [Google Scholar]
- 9.Punwaney R, Brandwein M, Zhang D, et al. Human papillomavirus may be common within nasopharyngeal carcinoma of Caucasian Americans: investigation of Epstein-Barr virus and human papillomavirus in eastern and western nasopharyngeal carcinoma using ligation-dependent polymerase chain reaction. Head Neck. 1999;21:21–29. [DOI] [PubMed] [Google Scholar]
- 10.Mirzamani N, Salehian P, Farhadi M, Tehran E. Detection of EBV and HPV in nasopharyngeal carcinoma by in situ hybridization. Exp Mol Pathol. 2006;81:231–234. [DOI] [PubMed] [Google Scholar]
- 11.Lo E, Bell D, Woo J, et al. Human papillomavirus and WHO type I nasopharyngeal carcinoma. Laryngoscope. 2010;120: 1990–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laantri N, Attaleb M, Kandil M, et al. Human papillomavirus detection in moroccan patients with nasopharyngeal carcinoma. Infect Agents Cancer. 2011;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singhi A, Califano J, Westra W. High-risk human papillomavirus in nasopharyngeal carcinoma. Head Neck. 2012;34:213–218. [DOI] [PubMed] [Google Scholar]
- 14.Dogan S, Hedberg M, Ferris R, Rath T, Assaad A, Chiosea S. Human papillomavirus and Epstein-Barr virus in nasopharyngeal carcinoma in a low-incidence population. Head Neck. 2014;36(4):511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson M, Suh Y, Paleri V, et al. Oncogenic human papillomavirus-associated nasopharyngeal carcinoma: an observational study of correlation with ethnicity, histological subtype and outcome in a UKpopulation. Infect Agents Cancer. 2013;8(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson D, Crandley E, Sim A, et al. Prognostic significance ofp16 and its relationship with human papillomavirus in pharyngeal squamous cell carcinomas. JAMA Otolaryngol Head Neck Surg. 2014;140(7):647–653. [DOI] [PubMed] [Google Scholar]
- 17.Stenmark M, McHugh J, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kano M, Kondo S, Wakisaka N, et al. The influence of human papillomavirus on nasopharyngeal carcinoma in Japan. Auris Nasus Larynx. 2017;44(3):327–332. [DOI] [PubMed] [Google Scholar]
- 19.Shi W, Pataki I, MacMilln C, et al. Molecular parameters in human nasopharyngeal carcinoma. Cancer. 2002;94(7):1997–2006. [DOI] [PubMed] [Google Scholar]
- 20.Young L, Dawson C. Epstein-Barr virus and nasopharyngeal carcinoma. Chin J Cancer. 2014;33(12):581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niedobitek G.Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Mol Pathol. 2000;53:248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann M, Ihloff A, Gorogh T, et al. p16(INK4a) overexpression predicts translational active human papillomavirus infection in tonsillar cancer. Int J Cancer. 2010;127(7): 1595–1602. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, Yang K, Khafagi A, et al. Sensitive detection of human papilomavirus in cervial, head/neck, and schistosomiasis-associated bladder malignancies. Proc Natl Acad Sci. 2005; 102(21):7683–7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C, Hsiao J, Yang M, et al. Human papilloma virusdetection in neoplastic and non-neoplastic nasopharyngeal tissues in Taiwan. J Clin Pathol. 2011;64(7):571–577. [DOI] [PubMed] [Google Scholar]
- 25.Atighechi S, Ahmadpour Baghdadabad M, Mirvakili S, et al. Human papilloma virus and nasopharyngeal carcinoma: pathology, prognosis, recurrence and mortality of the disease. Exp Oncol. 2014;36(3):215–216. [PubMed] [Google Scholar]
- 26.Fakhry C, Westra W, Wang S, et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Torabi S, Yarbrough W, Mehra S, Osborn H, Judson B. Association of human papillomavirus status at head and neck carcinoma subsites with overall survival. JAMA Otolaryngol Head Neck Surg. 2018;144(6):519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma V, Simone C, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696–706. [DOI] [PubMed] [Google Scholar]
- 29.Yip K, Shi W, Pintillie M, et al. Prognostic significance of the Epstein-Barr virus, p53, Bcl-2, and survivin in nasopharyngeal cancer. Clin Cancer Res. 2006;12(19):5726–5732. [DOI] [PubMed] [Google Scholar]
- 30.Lin Z, Khong B, Kwok S, et al. Human papillomavirus16 detected in nasopharyngeal carcinomas in white Americans but not in endemic southern Chinese patients. Head Neck. 2014;36(5):709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang W, Chamberlain P, Garden A, et al. Prognostic value ofp16 expression in Epstein-Barr virus-positive nasopharyngeal carcinoma. Head Neck. 2016;38(suppl):E1459–E1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng H, Chen L, Zhang Y, et al. Survival analysis of patients with advanced-stage nasopharyngeal carcinoma according to the Epstein-Barr virus status. Oncotarget. 2016;7(17): 24208–24216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/ p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32:562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu L, Li C, Pan L. Nasopharyngeal carcinoma: a review of current updates. Exp Ther Med. 2018;15(4):3687–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deschler D, Richmon J, Khariwala S, Ferris R, Wang M. The “new” head and neck cancer patient-young, nonsmoker, nondrinker, and HPV positive: evaluation. Otolaryngol Head Neck Surg. 2014;151(3):375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anantharaman D, Abedi-Ardekani B, Beachler D, et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int J Cancer. 2017;140(9): 1968–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torre V, Bucolo S, Giordano C, et al. Palatine tonsils in smokerand non-smoker patients: a pilot clinicopathological and ultrastructural study. J Oral Pathol Med. 2005;34(7):390–396. [DOI] [PubMed] [Google Scholar]
- 38.Giuliano A, Sedjo R, Roe D, et al. Clearance of oncogenic human papillomavirus (HPV) infection: effect of smoking (United States). Cancer Causes Control. 2002;13(9):839–846. [DOI] [PubMed] [Google Scholar]
- 39.Portugal L, Goldenberg J, Wenig B, et al. Human papillomavirus expression and p53 gene mutations in squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1997;123(11): 1230–1234. [DOI] [PubMed] [Google Scholar]
- 40.Burger M, Hollema H, Gouw A, Pieters W, Quint W. Cigarette smoking and human papillomavirus in patients with reported cervical cytological abnormality. BMJ. 1993;306(6880):749–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castellsague X, Monoz N. Chapter 3: cofactors in human papillomavirus carcinogenesis—role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003;31: 20–28. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.