Fig. 5.
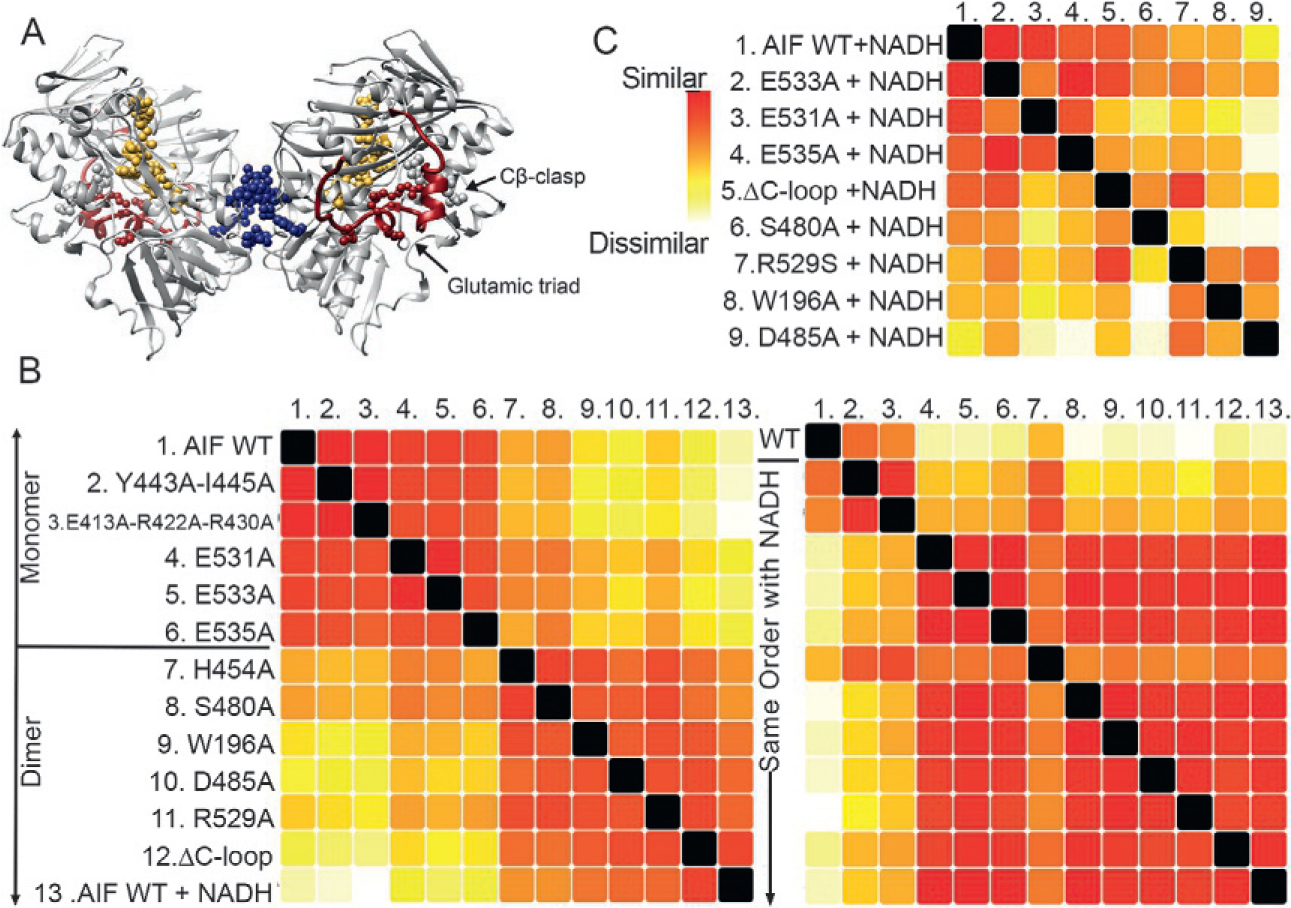
SAXS uncovers allosteric communication between a metabolite binding site and dimer interface. (A) The protein AIF binds the metabolite NADH at its central active site (gold), triggering dimerization at a distal surface (blue) and release of a surface C-loop (red). Allosteric residues targeted for mutation are shown as spheres. Dimeric AIF supports OXPHOS biogenesis, a target of interest for cancer treatment. (B) SSM analysis of AIF mutants relative to wild-type (WT) monomeric AIF without NADH (left panel) distinguishes AIF mutants consistent with the AIF monomer (top) from AIF mutants that dimerize without NADH (bottom). Complementary SSM analysis of NADH-saturated AIF mutants (right panel) suggest high similarity to the wild-type AIF dimer except for Y443A-I445A and E413A-R422A-R430A (defective for dimerization) and H454A (defective for C-loop release). (C) Extended SSM analysis of NADH-saturated AIF mutants relative to wild-type AIF dimer conformationally partitions mutants of AIF’s glutamic acid triad and Cβ-clasp.
