Table 3.
Ligand enabled C(sp2)─H hydroxylation of benzoic acidsa
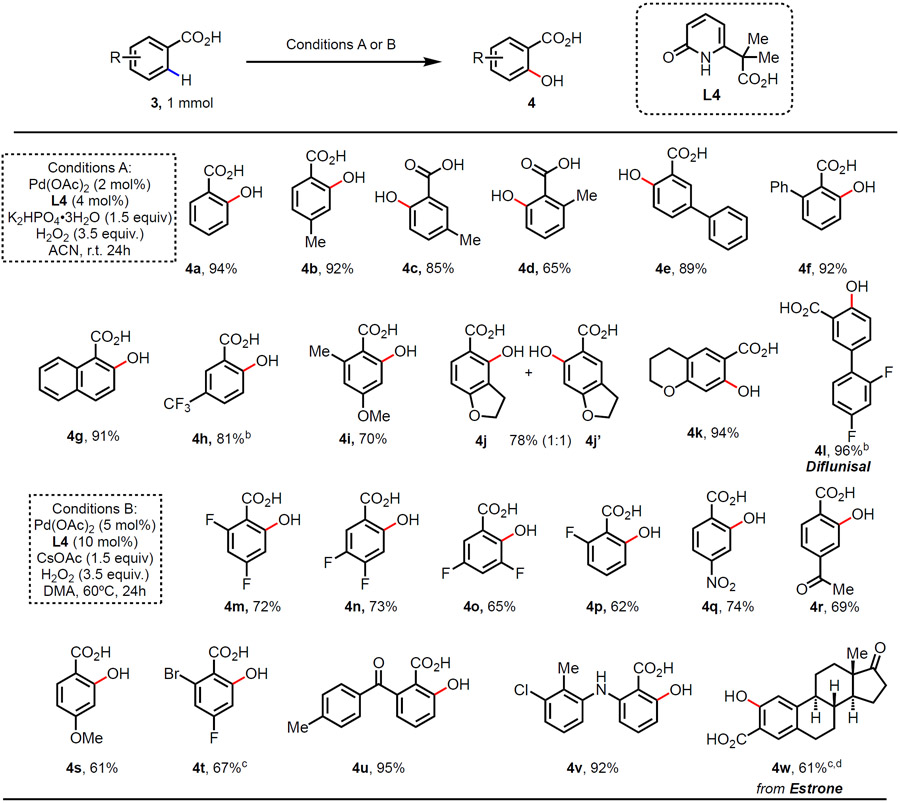
|
Conditions A: Carboxylic acid 3 (1 mmol), Pd(OAc)2 (2 mol%), L4 (4 mol%), H2O2 (35% aqueous solution, 3.5 equiv.), and K2HPO4•3H2O (1.5 equiv.) in CH3CN (3.0 mL), r.t. 24 h.; Conditions B: Carboxylic acid 3 (1 mmol), Pd(OAc)2 (5 mol%), 14 (10 mol%), H2O2 (35% aqueous solution, 3.5 equiv.), and CsOAc (1.5 equiv.) in DMA (3.0 mL), 60 °C. 24 h. Isolated yields.
60 °C
K2HPO4•3H2O instead of CsOAc, 48h.
0.5 mmol scale, r.t.
