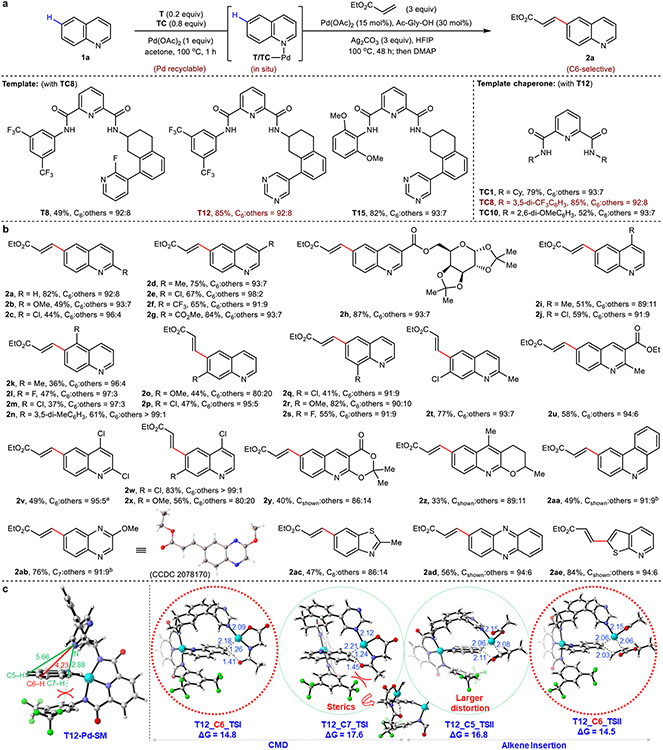
Fig. 2. C6 (and related)-selective C–H olefination reactions of quinoline and other heterocycles.
a, Selected optimization of directing template and template chaperone scaffolds. b, Scope of aza-arenes. c, DFT analysis rationalizes the observed C6 selectivity of quinoline 1a for template T12. Optimization yield and selectivity of 2a are determined by 1H NMR analysis. All yields are isolated yields for reaction scope. Bond lengths are denoted in Å. aUsing T8 (0.2 equiv). bUsing T15 (0.2 equiv) and TC10 (0.8 equiv). Cy, cyclohexyl; SM, starting material; TS, transition state.