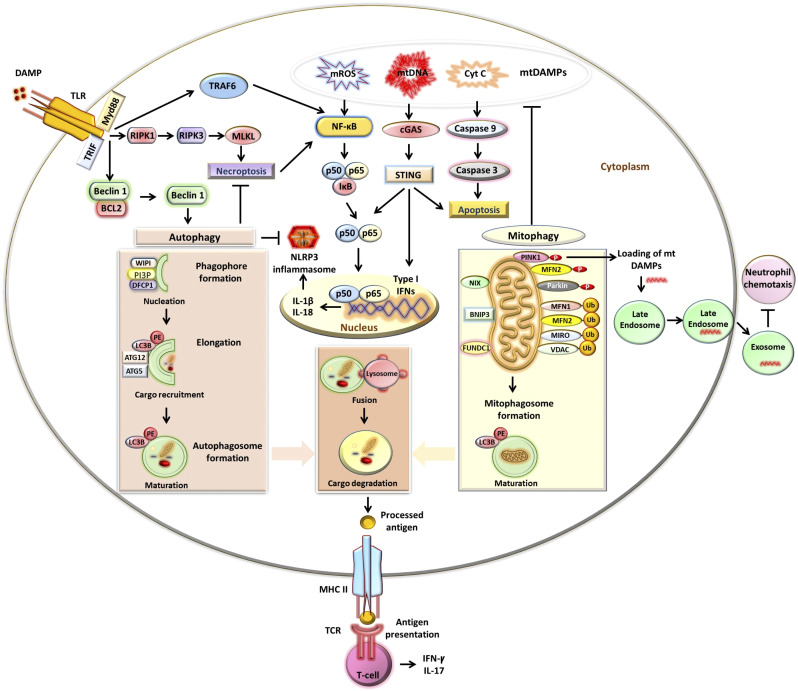
Figure 1.
Autophagy and mitophagy in immunity and inflammation. Autophagy is activated via the induction of Toll-like receptor (TLR) signaling. TLR binds to the damage-associated molecular pattern (DAMP) and adaptor proteins: myeloid differentiation primary response 88 (MyD88) and Toll-interleukin-1 receptor domain-containing adapter inducing interferon (IFN)-γ (TRIF) activate downstream signaling pathways. MyD88 and TRIF stimulate autophagy by suppressing the interaction between beclin 1 and B cell lymphoma 2 (BCL2) proteins. Autophagy involves engagement of phosphatidylinositol 3-phosphate (PI3P)-binding protein of WD-repeat protein interacting with phosphoinositide (WIPI) and double FYVE domain-containing protein 1 (DFCP1) with PI3P and phagophore formation. Autophagy-related genes (ATG5–ATG12) are conjugated by ATG7 and ATG10. ATG4B via its microtubule-associated protein light chain 3 (LC3)-interacting region (LIR) motif binds to LC3, cleaves, and generates LC3-I. ATG7 and ATG10 help in the conjugation of phospholipid phosphatidylethanolamine (PE) with unconjugated LC3-I and generate LC3-PE. LC3-PE binds with LIR-expressing autophagy substrate via p62/sequestosome1 (SQSTM1). The closed phagophore/autophagosome fuses with a lysosome to generate an autophagolysosome, which degrades cargo components. TLR signaling also activates receptor-interacting protein kinase (RIPK)1, resulting in RIPK3-mediated phosphorylation of mixed-lineage kinase domain-like pseudokinase (MLKL) and necroptosis. Autophagy inhibits necroptosis. Autophagy processes antigens and also participates in modulating T cell effector functions via major histocompatibility complex class II (MHC II)-dependent antigen presentation to the T cell receptor (TCR). Mitophagy involves the formation of a mitophagosome. Phosphatase and tensin homolog (PTEN)-induced kinase1 (PINK1) phosphorylates mitofusin (MFN)2, which recruits Parkin to the damaged outer mitochondrial membrane (OMM). Parkin polyubiquitinates (Ub) OMM proteins, including MFN1, MFN2, MIRO, and voltage-dependent anion channel (VDAC), which then bind with LC3-PE via p62. In PINK1-independent mitophagy, LC3-PE directly binds with OMM proteins: NIP3-like protein X (NIX), BCL2 adenovirus E1B 19 kDa-interacting protein 3 (BNIP3), and FUN14 domain-containing 1 (FUNDC1), through LIR. Mitophagy by inhibiting the release of mitochondrial DAMPs (mtDAMPs), including mitochondria-derived reactive oxygen species (mROS), mitochondrial DNA (mtDNA), and cytochrome c (Cyt C), suppresses the production of type I IFNs and inflammatory cytokines. TLR signaling also activates the NF-κB signaling pathway, through tumor necrosis factor receptor-associated factor 6 (TRAF6), production of inflammatory cytokines, and formation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome. Autophagy inhibits NLRP3 inflammasome formation. The mitophagy mediator PINK1 also facilitates the loading of mtDAMPs to the late endosome. Endosome-dependent release of mtDAMPs prevents neutrophil chemotaxis.