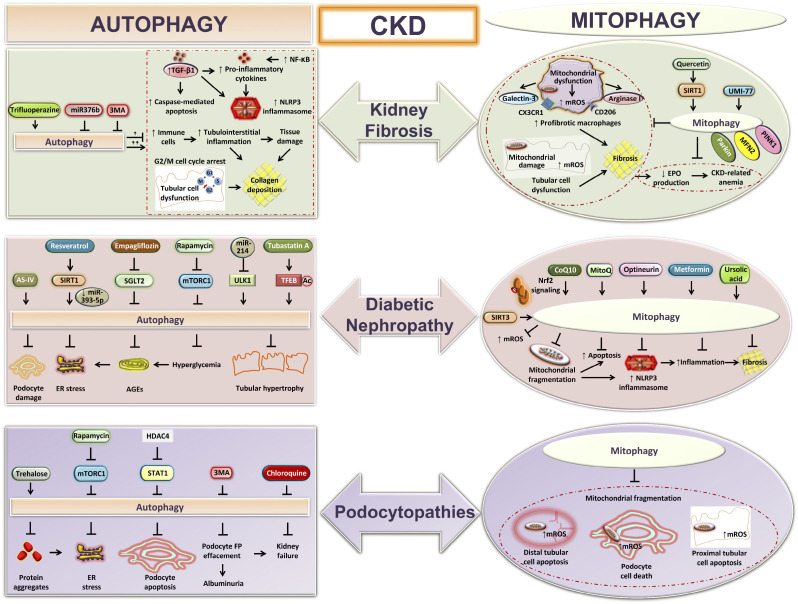
Figure 3.
Autophagy and mitophagy during chronic kidney disease (CKD). Shown is a graphical presentation showing the functions of autophagy and mitophagy in different models of CKD: kidney fibrosis, diabetic nephropathy, and podocytopathies. Autophagy prevents tubulointerstitial inflammation, tubular hypertrophy, loss of podocytes, and kidney fibrosis during CKD. Excessive autophagy can also exaggerate tubular injury and tubulointerstitial fibrosis. Mitophagy attenuates kidney macrophage-derived fibrotic responses, podocyte-, and tubular cell-derived mitochondria-specific oxidative stress, associated cell death, collagen deposition, and CKD-related anemia. AGEs, advanced glycation end products; AS-IV, astragaloside IV; CoQ10, coenzyme Q10; EPO, erythropoietin; ER, endoplasmic reticulum; FP, foot process; HDAC4, histone deacetylase-4; MFN2, mitofusin 2; MitoQ, mitoquinone; mROS, mitochondria-derived reactive oxygen species; mTORC1, mechanistic target of rapamycin (mTOR) kinase complex 1; NLRP3, NLR family pyrin domain containing 3; Nrf2, nuclear erythroid 2-related factor 2; PINK1, phosphatase and tensin homolog (PTEN)-induced kinase1; SGLT2, Na+-glucose cotransporter-2; SIRT, sirtuin; STAT1, signal transducer and activator of transcription 1; TFEB, transcription factor EB; TGF-β1, transforming growth factor-β1; 3MA, 3-methyladenine; ULK1, uncoordinated-51-like protein kinase 1.