Abstract
Rationale & Objective:
Sodium-glucose co-transporter 2 inhibitors (SGLT2i) are recommended for type 2 diabetes mellitus (T2DM) in patients with chronic kidney disease (CKD) or atherosclerotic cardiovascular disease (ASCVD). We evaluated factors associated with SGLT2i prescription, disparities by race and sex, and facility-level variation in prescription patterns.
Study Design:
Retrospective cohort.
Setting & Participants:
A national sample of U.S. veterans with comorbid T2DM, CKD, and ASCVD with a primary care visit between January 1 and December 31, 2020.
Exposures:
Race, sex, and individual VA location.
Outcome:
SGLT2i prescription.
Analytical Approach:
Multivariable logistic regression assessed associations of race and sex with SGLT2i prescription. Facility-level variation in SGLT2i prescription was quantified by median rate ratios (MRR), which express the likelihood that two randomly selected facilities differ in their use of SGLT2i among similar patients.
Results:
Of 174,443 patients with CKD, T2DM, and ASCVD, 20,024 (11.5%) were prescribed an SGLT2i. Lower odds of SGLT2i prescription were seen in Black or African American patients compared to White patients, odds ratio (OR) 0.87 (95% confidence interval [CI] 0.83-0.91), and among women compared to men, OR 0.59 (95% CI 0.52-0.67). The adjusted MRR for SGLT2i prescription was 1.58 (95% CI 1.48-1.67) in the total cohort, indicating an unexplained 58% variation in treatment between VA facilities, independent of patient and facility characteristics. Facility-level variation was evaluated among Black or African American patients (MRR 1.55 [95% CI 1.41-1.68]), White patients (MRR 1.57 [95% CI 1.47-1.66]), women (MRR 1.40 [95% CI 1.28-1.51]), and men (MRR 1.57 [95% CI 1.48-1.67]).
Limitations:
Albuminuria was not assessed.
Conclusions:
Prescription for SGLT2i was low among likely eligible patients, with evident disparities by sex and race and between individual VA facilities. Efforts are needed to study and address the reasons for these disparities to improve equitable adoption of these important medications.
Keywords: SGLT2 inhibitors, chronic kidney disease, diabetes mellitus, atherosclerotic cardiovascular disease
Introduction
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) have been recommended for type 2 diabetes mellitus (T2DM) in patients with chronic kidney disease (CKD) with an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2 since 2018 based on cardiovascular benefits seen in large clinical trials. 1-3 Evidence for their use in patients with CKD strengthened in 2019 with publication of the Canagliflozin and Renal Events in Diabetes with Established Nephropathy (CREDENCE) Trial, which showed a reduction in kidney endpoints in patients with CKD stages 2-3 who were randomized to canagliflozin compared to placebo.4 This and other accumulated evidence has since led to their incorporation into guidelines as first line therapy for individuals with T2DM and CKD.5
In the setting of the increasing body of evidence supporting their benefits, SGLT2i prescription was adopted in the Veterans Affairs (VA) Health Care System for individuals with T2DM and comorbid CKD, atherosclerotic cardiovascular disease (ASCVD), or heart failure with reduced ejection fraction. Many patients receiving VA care, therefore, have access to these prescriptions without concern for high out-of-pocket cost. Nevertheless, studies show that these agents are underutilized,6-8 likely due to multiple barriers related to guidelines, clinicians, and patient-level factors, as has been seen with other medication classes.9 Recent studies have identified racial and ethnic disparities in SGLT2i prescription that may contribute to underutilization.10 We sought to evaluate factors associated with prevalent SGLT2i prescription and disparities in SGLT2i prescription patterns by race and sex, as well as facility-level variation in prescription patterns among patients with comorbid CKD, T2DM, and ASCVD, for whom these medications were likely indicated.
METHODS
Data Source and Participants
Using national data from the U.S. VA Corporate Data Warehouse, we identified adult patients with CKD, T2DM, and ASCVD across 130 VA locations and their affiliated outpatient clinics who had an in-person or telehealth primary care provider (PCP) visit (index visit) between January 1, 2020 and December 31, 2020. For individuals with multiple PCP visits during this window, the most recent visit was used as the index visit for the study. CKD was defined as a baseline eGFR <60 mL/min/1.73 m2. T2DM was ascertained by International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis code, hemoglobin A1c ≥6.5%, fasting plasma glucose ≥126 mg/dL, random plasma glucose ≥200 mg/dL, or the use of diabetes medications within 2 years prior to the index visit. ASCVD was identified using ICD-10-CM, Current Procedural Terminology (CPT), and procedure codes. Individuals with eGFR <30 mL/min/1.73 m2 or type 1 diabetes mellitus were excluded, consistent with VA criteria for SGLT2i prescription at that time.
The VA Pharmacy Benefits Manager publishes Criteria for Use for restricted medications such as SGLT2is. These criteria are applicable nationally across the VA system. From June 2019 to November 2020, empagliflozin, the formulary SGLT2i for the VA health care system, was permitted to be prescribed for individuals with T2DM who were already receiving or were unable to receive metformin, and at least one of ASCVD, heart failure with reduced ejection fraction, or CKD, defined as an eGFR 30 to 59 mL/min/1.73 m2 or a urine albumin-to-creatinine ratio (UACR) ≥30 mg/g. Exclusion criteria included a history of serious hypersensitivity to an SGLT2i, dialysis-dependence, pregnant or nursing, pancreatic disorders suggestive of insulin deficiency, a history of frequent urinary tract infections (UTI) or risk for UTI, or a hemoglobin A1c >10%. Based on emerging data, in November 2020 the criteria were expanded to include any individual with T2DM already receiving or unable to receive metformin. This update also included that empagliflozin could be initiated for any individual with or without T2DM with an eGFR ≥25 mL/min/1.73 m2, UACR ≥200 mg/g, and on maximally tolerated angiotensin converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) therapy. Exclusion criteria included those on dialysis; pregnant or nursing; type 1 diabetes mellitus or factors predisposing to ketoacidosis; or a history of frequent genital mycotic infections, UTI, or risk factors for UTI. Based on these established, nationally applicable criteria for use in the VA system, during the time of data capture, individuals with comorbid T2DM, ASCVD, and CKD had multiple indications for SGLT2i therapy and would have been eligible to receive SGLT2i in the absence of a contraindication. Prescriptions for SGLT2i would not have been restricted by the health care system for this patient population during the time of data capture.
The study was approved by the institutional review board at Baylor College of Medicine and the Michael E. DeBakey VA Medical Center. A waiver of informed consent was granted.
SGLT2i Use
Empagliflozin is the formulary SGLT2i for the VA system, but individuals who do not tolerate formulary agents and still require treatment can receive approval for a non-formulary alternative. Some veterans also receive medications outside the VA system, which are documented as non-VA medications and were captured in our dataset. SGLT2i exposure was identified by pharmacy records, defined as any prescription for canagliflozin, dapagliflozin, empagliflozin, or ertugliflozin within 180 days before or 100 days after the index visit. Both SGLT2i prescriptions generated in the VA system and those documented as non-VA prescriptions were captured to define SGLT2i exposure.
Covariates
We obtained patient characteristics including age, demographic information, and body mass index (BMI). Comorbidities were identified by ICD-10-CM codes. Laboratory values including creatinine and hemoglobin A1c were ascertained. Baseline eGFR was calculated from the creatinine value most recently obtained within one year prior to the index visit using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.11 Pharmacy records were used to obtain concomitant medication prescriptions for other glucose lowering medications and for other cardioprotective medications that are frequently indicated in this patient population, including any statin therapy, high-intensity statin therapy, and ACEi or ARB use. Variables related to health care delivery were also obtained, including whether the PCP of the index visit was a physician vs. an advanced practice provider such as a nurse practitioner or physician assistant, and whether the index visit occurred at a teaching facility. The number of visits in the 12 months prior to the index visit with primary care, cardiology, endocrinology, and nephrology were captured to represent patient complexity and ascertain involvement of pertinent specialists who might have prescribed these medications.
Statistical Analysis
Descriptive statistics compared baseline characteristics between those prescribed and not prescribed an SGLT2i. Categorical variables were compared using Chi-square or Fisher’s exact tests, and continuous variables were compared using Student’s t tests for normally distributed variables and Kruskal-Wallis tests for non-normally distributed variables. Multivariable logistic regression assessed associations between baseline characteristics with SGLT2i prescription, adjusting forage, sex, race, hypertension, systolic heart failure, ischemic heart disease, BMI, eGFR, hemoglobin A1c, concomitant medication use, physician vs. advanced practice provider as PCP, receipt of care at a teaching facility, and the number of visits within 12 months prior to the index visit with PCP, cardiology, endocrinology, and nephrology. Disparities in SGLT2i prescription by race (Black or African American vs. White) and sex (women vs. men) were further evaluated by multivariable logistic regression among subgroups, including by age, sex, race, comorbidities (hypertension, heart failure, ischemic heart disease, peripheral arterial disease, and ischemic cerebrovascular disease), BMI, eGFR, hemoglobin A1c, physician PCP, and receipt of care at a teaching hospital. Facility-level variation in SGLT2i prescription was quantified by a median rate ratio (MRR), which expresses the likelihood that two randomly selected facilities differ in their use of SGLT2i among similar patients. MRR were calculated using generalized linear models for the entire cohort and by subgroups defined by race and sex. Facilities with fewer than 10 patients with CKD, T2DM, and ASCVD were excluded from this analysis. Analyses were done using STATA 16 (College Station, TX) and SAS 9.4.
RESULTS
Baseline Characteristics
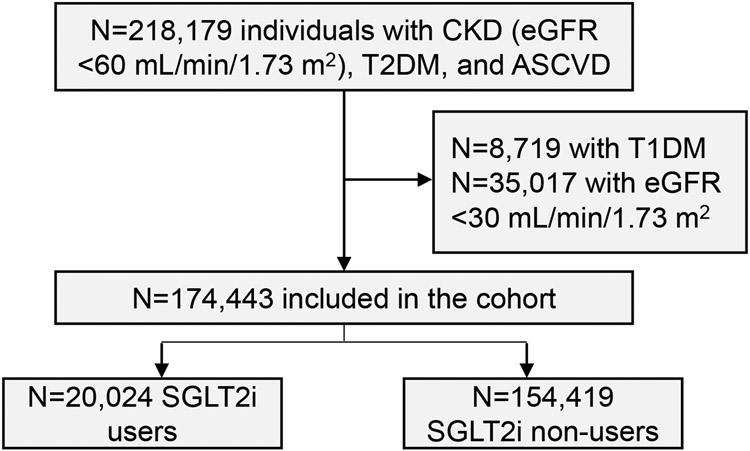
We identified 174,443 patients with CKD, T2DM, and ASCVD. Of those, 20,024 (11.5%) were prescribed an SGLT2i (Figure 1). Those prescribed an SGLT2i were younger, mean (SD) 72.0 (6.9) vs. 75.9 (8.1) years, P<0.001 (Table 1). The cohort was male-predominant, with 19,648 (98.3%) males in the SGLT2i user group and 151,296 (98.0%) in the SGLT2i non-user group, P=0.002. Race differed between the two groups, with SGLT2i users comprised of 80.0% White and 12.3% Black or African American individuals, while the non-SGLT2i user group included 79.7% White and 12.6% Black or African American patients, P<0.001. SGLT2i users were more likely to have systolic heart failure (26.5% vs. 20.3%) and ischemic heart disease (86.3% vs. 80.7%), but less likely to have peripheral arterial disease (23.7% vs. 26.5%) and ischemic cerebrovascular disease (25.8% vs. 28.4%), P<0.001 for each. They were more likely to be prescribed statins, high-intensity statins, insulin, biguanides, thiazolidinediones, dipeptidyl-peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists, ACEi or ARB, and beta blockers (P<0.001 for each, Table 1). They had higher hemoglobin A1c, mean (SD) 8.0% (1.4) vs. 7.3% (1.4), higher BMI, mean (SD) 32.4 (6.1) vs. 31.0 (6.1) kg/m2, and higher eGFR, mean (SD) 48.5 (7.6) vs. 47.2 (8.2) mL/min/1.73 m2, P<0.001 for each. SGLT2i users were also more likely to receive care at a teaching facility and have a physician as their PCP, P<0.001 for each.
Figure 1. Creation of the study cohort.
Table 1.
Baseline characteristics by SGLT2i use
| Characteristics | SGLT2i users, n=20,024 |
SGLT2i non-users, n=154,419 |
|---|---|---|
| Age, mean (SD) | 72.0 (6.9) | 75.9 (8.1) |
| Male sex, n (%) | 19,684 (98.3%) | 151,296 (98.0%) |
| Race, n (%) | ||
| White | 16,024 (80.0%) | 123,057 (79.7%) |
| Black or African American | 2,463 (12.3%) | 19,462 (12.6%) |
| Native Hawaiian or Pacific Islander | 235 (1.2%) | 1,540 (1.0%) |
| American Indian or Alaska Native | 117 (0.9%) | 1,346 (0.9%) |
| Asian | 169 (0.8%) | 923 (0.6%) |
| Undefined, other, or mixed race | 956 (4.8%) | 8,091 (5.2%) |
| Comorbidities, n (%) | ||
| Hypertension | 19,004 (94.9%) | 143,158 (92.6%) |
| Systolic heart failure | 5,314 (26.5%) | 31,397 (20.3%) |
| Ischemic heart disease | 17,282 (86.3%) | 124,634 (80.7%) |
| Peripheral arterial disease | 4,739 (23.7%) | 40,878 (26.5%) |
| Ischemic cerebrovascular disease | 5,164 (25.8%) | 43,860 (28.4%) |
| Medications, n (%) | ||
| Statins | ||
| Any statin | 18,724 (93.5%) | 134,675 (87.2%) |
| High-intensity statin | 13,355 (66.7%) | 78,076 (50.6%) |
| Glucose lowering medications | ||
| Sulfonylurea | 6,265 (31.3%) | 36,406 (23.5%) |
| Insulin | 12,255 (61.2%) | 60,583 (39.2%) |
| Biguanide | 11,824 (59.1%) | 57,170 (37.0%) |
| Thiazolidinedione | 998 (5.0%) | 4,149 (2.7%) |
| DPP-4 inhibitor | 3,523 (17.6%) | 15,374 (10.0%) |
| GLP-1 receptor agonist | 4,372 (21.8%) | 12,445 (8.0%) |
| Anti-hypertensive agents | ||
| ACE inhibitor or ARB | 14,906 (74.4%) | 91,047 (59.0%) |
| Beta-blocker | 14,770 (73.8%) | 93,005 (60.2%) |
| Body mass index in kg/m2, mean (SD) | 32.4 (6.1) | 31.0 (6.1) |
| <18.5 kg/m2, n (%) | 31 (0.2%) | 718 (0.5%) |
| 18.5-24.9 kg/m2, n (%) | 1,659 (8.3%) | 21,975 (14.3%) |
| 25-29.9 kg/m2, n (%) | 5,937 (29.7%) | 51,438 (33.5%) |
| ≥30 kg/m2, n (%) | 12,338 (61.8%) | 79,513 (51.7%) |
| Estimated GFR, mean (SD) | 48.5 (7.6) | 47.2 (8.2) |
| 45-59 mL/min/1.73 m2, n (%) | 6,375 (31.8%) | 59,634 (38.6%) |
| 30-44 mL/min/1.73 m2, n (%) | 13,649 (68.2%) | 94,785 (38.6%) |
| Hemoglobin A1c, mean (SD) | 8.0 (1.4) | 7.3 (1.4) |
| ≤7.0%, n (%) | 4,481 (22.9%) | 76,467 (52.6%) |
| >7.0-8.0%, n (%) | 6,827 (34.9%) | 37,500 (25.8%) |
| >8.0-9.0%, n (%) | 4,665 (23.8%) | 17,487 (12.0%) |
| >9.0%, n (%) | 3,601 (18.4%) | 14,025 (9.6%) |
| Physician primary care provider, n (%) | 15,350 (76.7%) | 116,005 (75.1%) |
| Receipt of care at a teaching facility, n (%) | 7,050 (35.2%) | 50,950 (33.0%) |
| Number of visits in the 12 months prior to the index visit, mean (SD) | ||
| Primary care provider | 10.4 (7.2) | 7.9 (6.4) |
| Cardiology | 1.3(2.8) | 0.8 (2.0) |
| Endocrinology | 0.5 (1.4) | 0.2 (0.9) |
| Nephrology | 0.3 (1.0) | 0.3 (1.0) |
Abbreviation: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; DPP4, dipeptidyl-peptidase 4; GFR, glomerular filtration rate; GLP-1, glucagon-like peptide 1; IQR, interquartile range; SD, standard deviation; SGLT2i, sodium-glucose co-transporter-2 inhibitor Due to the large sample size, all P values were highly statistically significant with P<0.001, with the exception of sex (P=0.002). Comparisons between groups were made using Chi square tests or Fisher’s exact tests for categorical variables and Student’s t tests for normally distributed or Kruskal-Wallis tests non-normally distributed continuous variables.
Factors Associated with SGLT2i Use
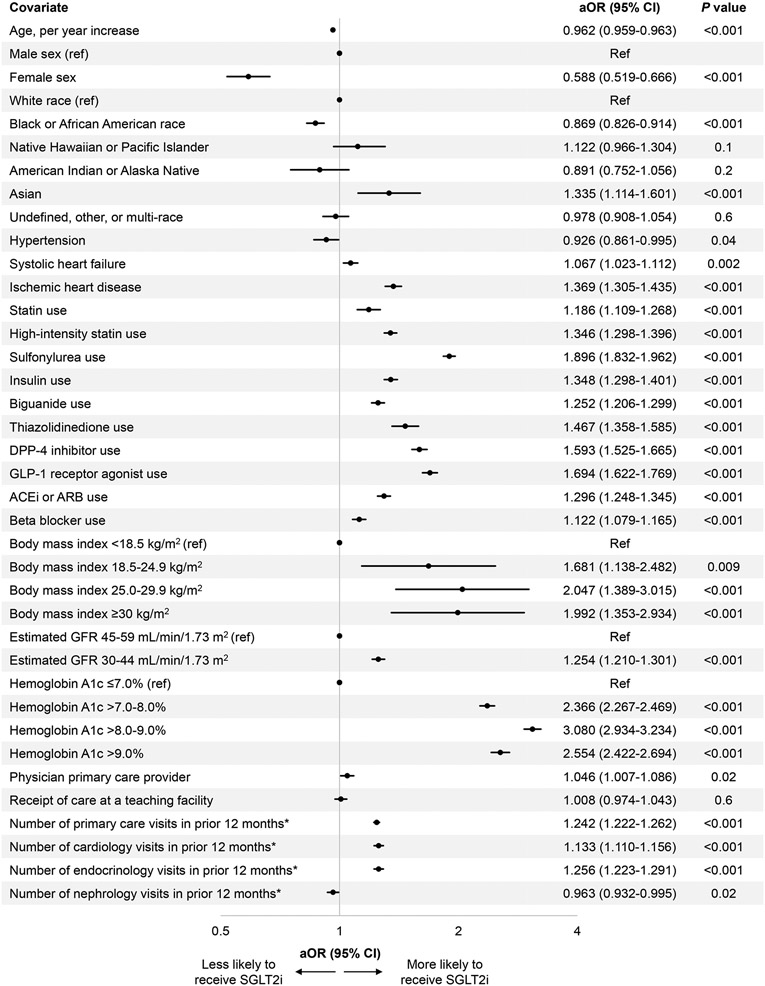
In the multivariable model, Black or African American patients were less likely to be prescribed an SGLT2i compared to White patients, adjusted odds ratio (aOR) (95% confidence interval [CI]) 0.87 (0.83, 0.91), P<0.001, and women were almost half as likely to be prescribed an SGLT2i compared to men, aOR (95% CI) 0.59 (0.52, 0.67), P<0.001 (Figure 2). Younger age, ischemic heart disease, use of concomitant medications, higher BMI, higher hemoglobin A1c, and higher number of visits with PCP, cardiology, or endocrinology were associated with higher odds of SGLT2i prescription. Lower odds of SGLT2i prescription were seen in individuals with more nephrology visits in the preceding 12 months.
Figure 2. Factors associated with SGLT2i prescription.
A Forest plot displays the multivariable logistic regression analyses for the odds of SGLT2i prescription based on patient demographic and clinical characteristics among patients with CKD, T2DM, and ASCVD. The model is adjusted for age; sex; race; hypertension; systolic heart failure; ischemic heart disease; use of statins, high-intensity statins, sulfonylurea, insulin, biguanide, thiazolidinedione, dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) agonist, angiotensin converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB), or beta blocker; body mass index (BMI); estimated glomerular filtration rate (eGFR); hemoglobin A1c; whether the primary care provider (PCP) was a physician or advanced practice provider; whether care was received at a teaching facility; and the number of visits in the 12 months preceding the index visit with PCP, cardiology, endocrinology, and nephrology.
*Square root transformation was applied to address the right-skewed distribution of the number of visits within the 12 months prior to the index visit.
Race and sex disparities within subgroups
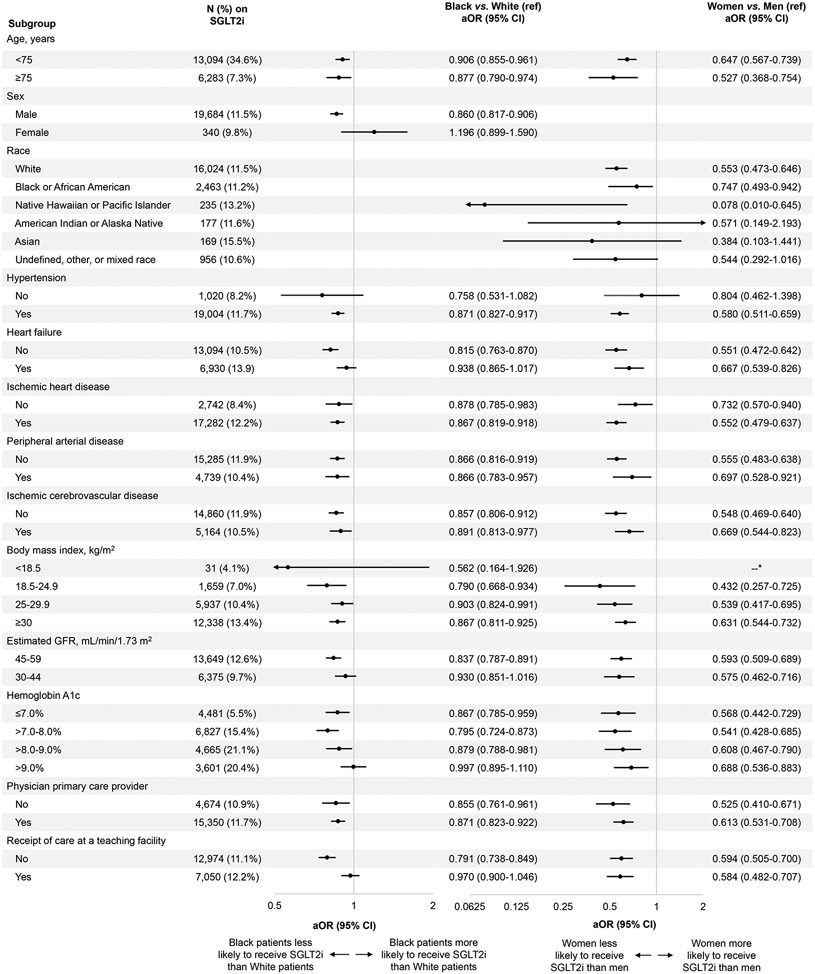
Further evaluation of disparities by race revealed that Black or African American patients were less likely than White patients to receive an SGLT2i among most subgroups by age, sex, comorbidities, or clinical characteristics (Figure 3). There was no difference between Black or African American and White patients among women, those without hypertension, with heart failure, with BMI <18.5 kg/m2, with eGFR 30-44 mL/min/1.73 m2, with hemoglobin A1c >9%, or who were receiving care at a teaching facility. All remaining subgroups showed that Black or African American patients had lower odds of SGLT2i prescription than White patients. Women were less likely than men to receive an SGLT2i among all subgroups, with the exceptions of racial groups other than White and Black or African American and those without hypertension (Figure 3).
Figure 3. Disparities in SGLT2i prescription by race and sex.
Analysis by the subgroups listed in the lefthand column shows the odds of SGLT2i prescription in Black or African American individuals compared to White individuals (referent) and in women compared to men (referent).
*There were no female SGLT2i users with a BMI <18.5 kg/m2.
Facility-Level Variation
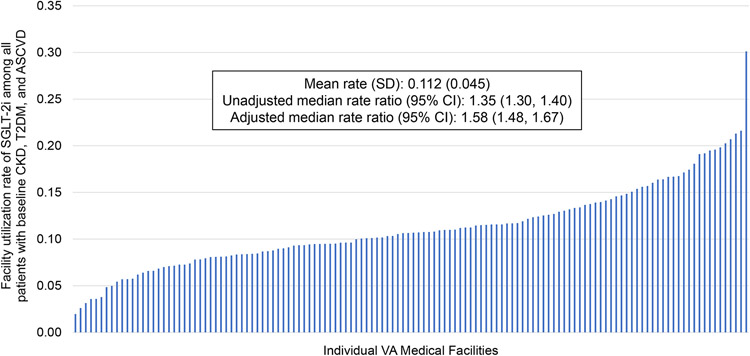
Across individual VA facilities, SGLT2i prescription rates varied, with a mean (SD) facility-level rate of 11.2% (4.5) of patients prescribed an SGLT2i. The crude MRR (95% CI) was 1.35 (1.30, 1.40). When adjusting for covariates, the MRR (95% CI) was 1.58 (1.48, 1.67) indicating a residual 58% variation in treatment with SGLT2i for two similar patients treated at two random facilities (Figure 4).
Figure 4. Facility-level variation in SGLT2i prescription.
SGLT2i prescription rates varied significantly between individual VA medical facilities in the entire cohort.
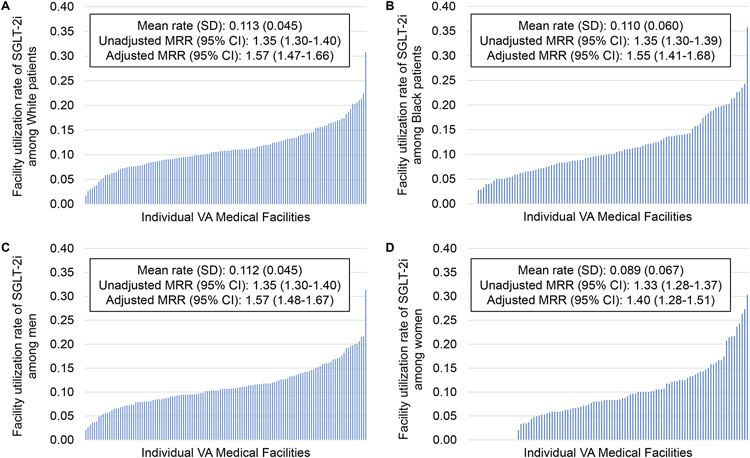
Facility-Level Variation by Race and Sex
All facilities had at least 10 White patients meeting inclusion criteria, so all were included for this subgroup. There were 22 facilities excluded from the analysis of Black or African American patients for having fewer than 10 patients in this group. Mean (SD) SGLT2i utilization rate was 11.3% (4.5) among White patients and 11.0% (6.0) among Black or African American patients (Figure 5A-B). The adjusted MRR (95% CI) was 1.57 (1.47, 1.66) among White patients and 1.55 (1.41, 1.68) among Black or African American patients. All facilities included in the study had at least 10 men meeting inclusion criteria and were included, but 22 VA locations were excluded from the analysis of women for having fewer than 10 women with T2DM, CKD, and ASCVD. Mean (SD) utilization rate was 11.2% (4.5) among men and 8.9% (6.7) among women (Figure 5C-D). The adjusted MRR (95% CI) was 1.57 (1.48, 1.67) among men and 1.40 (1.28, 1.51) among women. Of facilities with at least 10 patients meeting inclusion criteria, there were four facilities at which zero Black or African American patients and 19 at which no women in this study were prescribed an SGLT2i.
Figure 5. Facility-level variation in SGLT2i prescription by race and sex.
Facility-level variation is shown for individuals of White race (A), Black or African American race (B), men (C), and women (D).
DISCUSSION
In this analysis we demonstrated that SGLT2i utilization was low at only 11.5% of likely eligible patients with comorbid CKD, T2DM, and ASCVD. Black or African American patients and women were less likely overall to receive an SGLT2i prescription, which was largely consistent across subgroups. There was substantial variation in SGLT2i prescription patterns between VA facilities, with an unexplained 58% difference in the probability of similar patients receiving an SGLT2i at two random facilities.
Several factors were significantly associated with SGLT2i prescription. Patients receiving SGLT2i prescriptions were more likely to also receive a statin, beta blocker, and ACEi or ARB. This could indicate that SGLT2i recipients were seeing clinicians who were more likely to practice consistent with updated guideline recommendations. These relationships were independent of heart failure, which would necessitate guideline-directed medical therapy, and other markers of patient complexity such as the number of clinician visits in the year prior to the index date. Patients with more PCP, endocrinology, and cardiology visits were more likely to receive an SGLT2i, possibly due to indications for these agents in patients with cardiovascular disease or poorly controlled T2DM. However, patients with more nephrology visits were less likely to receive them. This may be due to hesitancy to prescribe these agents in individuals with CKD given the known phenomenon of the eGFR dip that occurs after initiation, despite the long-term protective effects of SGLT2i on kidney outcomes that was known at the time from the CREDENCE Trial.4 It should be noted that other major trials showing a benefit of SGLT2i therapy in patients with CKD and additional research studying the eGFR dip were published in late 2020 and afterwards, which has expanded our understanding of the safety of these agents in patients with CKD.12-14 Consequently, at the time of the data capture for our study, nephrologists may not yet have been commonly prescribing these medications, as the benefits in patients with CKD had yet to be replicated and incorporated into nephrology guidelines.5,15 This highlights the critical role PCPs and nephrologists can play to improve kidney outcomes by appropriately prescribing these agents for patients with CKD to avoid clinical inertia and underutilization, as has plagued the prescription of ACEis and ARBs.16
There were also evident racial and sex disparities in SGLT2i prescription, with Black or African American patients and women less likely to receive them. This finding has been previously demonstrated in other patient populations. A recent analysis evaluated all patients with T2DM in the VA system between January 1, 2019 and December 31, 2020.10 They found that SGLT2is were prescribed in 8.8% of Black or African American patients, compared to 11.0% among American Indian or Alaska Native patients, 11.8% among patients of Asian, Native Hawaiian, or Other Pacific Islander descent, and 11.3% of White patients. Adjusted models showed that American Indian or Alaska Native, Black or African American, multiracial, and unknown race subgroups had lower odds of SGLT2i prescription than White patients. Detailed subgroup analyses comparing prescription rates showed that Black or African American patients were less likely than White patients to be prescribed an SGLT2i in all subgroups by demographic characteristics, comorbidities, eGFR, albuminuria, hemoglobin A1c, or geographic region. Another study evaluated adults in the National Health and Nutrition Examination Survey (NHANES) and showed that from 2017-2020, 5.8% of individuals were prescribed an SGLT2i overall, with a weighted percentage of 3.6% (95% CI 2.3, 5.5) among non-Hispanic Black participants and 7.2% (4.1, 12.6) among non-Hispanic White participants, although this did not reach statistical significance (P=0.27).17 Their data also suggested a possible sex disparity, with SGLT2i prescription prevalence of 7.9% (95% CI 4.9, 12.4) in men compared to 3.5% (1.4, 8.2) in women (P=0.07). Sex disparities may be attributable in part due to exclusion criteria related to a history of recurrent UTI, which is more likely among women than men and is difficult to accurately ascertain using retrospective datasets. Our findings of racial disparities are consistent with prior literature and extend these findings to medically complex individuals with comorbid T2DM, CKD, and ASCVD who had multiple indications for SGLT2i prescription and fewer barriers such as access to care and prescription drug cost by receiving care within the VA system.6,18
We further found that the SGLT2i prescription patterns differed by individual VA location. Our results are consistent with prior studies in other patient populations demonstrating high variation in prescription prevalence between locations or geographic regions.19,20 VA Pharmacy Benefits Manager Criteria for Use are applicable at the national level to all included VA facilities, although local pharmacy management at individual VAs can further restrict medications beyond these criteria. In the VA system, access to care and affordability of medications are much lower barriers compared to other health systems in the United States, so the variation between facilities is likely independent of these important factors. The MRR seen across VA facilities augmented after adjustment for patient characteristics and facility variables. Ultimately this variability may be explained by unmeasurable phenomena at the level of individual clinicians, such as their familiarity with indications and contraindications, their understanding of the benefits seen in clinical trials, and their confidence regarding recognizing and appropriately managing adverse effects, suggesting the need for additional education about the benefits of these agents. Although we anticipate that overall SGLT2i utilization will increase over time with newer evidence and expanded guideline recommendations for patients with kidney disease,5,15 this variability between facilities indicates a system-level variable that needs to be addressed.
We also noted that although facility-level variation was similar between Black or African American patients and White patients, demographics, comorbidities, and clinical variables accounted for more variation seen among women than men. These analyses included only facilities that cared for at least 10 patients with T2DM, CKD, and ASCVD who were potentially eligible for SGLT2i treatment within the specified group. In other words, facilities that treated fewer than 10 women meeting inclusion criteria for the cohort were excluded from the subgroup analysis of facility-level variation among women. It was notable to identify four facilities at which no Black or African American patients in this cohort were prescribed an SGLT2i, and 19 facilities for which no women in this cohort received SGLT2i. This raises concerns for inequitable access to these important medications at some centers, so identifying and addressing system-level factors contributing to this may improve SGLT2i utilization.
Our study has several strengths. Using real-world prescription data, we were able to robustly describe prescription patterns nationally and by individual VA locations. By evaluating active users of the VA system who would highly likely meet eligibility criteria for SGLT2i prescription, our findings represent factors independent of access to care and cost that may affect SGLT2i utilization. Evaluating facility-level variation in this patient population and by race and sex subgroups is novel and demonstrated critical results. There are also important limitations worth mentioning. First, albuminuria was not ascertained. This is an important risk factor for the progression of CKD and may impact clinical decision making about the prescription of an SGLT2i. Second, we had only one measurement of eGFR prior to the index visit, so were unable to assess rate of eGFR decline. Third, the data regarding the benefits of SGLT2i have rapidly evolved over the last several years, but clinical data from 2021 were not available for analysis. Fourth, because we were not able to assess all SGLT2i exclusion criteria such as a history of recurrent UTIs, some participants in our study would have been ineligible for prescription. Thus, our estimates may underestimate utilization in patients who meet all eligibility criteria, although we anticipate that this would apply to a minority of patients.
In summary, prescription rate of SGLT2i for likely eligible patients was low, with substantial variation between individual facilities. Ischemic heart disease, concomitant medication prescription, higher BMI, and higher hemoglobin A1c were independently associated with SGLT2i prescription. There were evident racial and sex disparities in SGLT2i prescription that persisted among subgroups. Reasons underlying racial and sex disparities must be identified and addressed to ensure equitable access to these important medications. Further health services research should address barriers to SGLT2i prescription to increase guideline-based practice to improve long-term cardiovascular and kidney outcomes in patients with CKD.
Support:
This work was supported by a Department of Veterans Affairs Health Service Research & Development Service Investigator Initiated Grants (IIR 16-072, IIR 19-069), and the Houston VA Health Services Research & Development Center for Innovations grant (CIN13-413) and NIH/NHLBI grant K24 HL161414 (SDN). Support for VA/CMS data provided by the Department of Veterans Affairs, VA Health Services Research and Development Service, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004). Dr. Gregg is supported by a VA Clinical Sciences Research & Development Career Development Award (IK2CX002368). Dr. Virani is supported by research grants from the Department of Veterans Affairs, NIH, and the Tahir and Jooma Family. The funders did not have a role in study design, data collection, analysis, reporting or the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr. Navaneethan reports receiving personal fees from ACI clinical, Astrazeneca (Data safety monitoring board) Bayer, Boehringer Ingelheim and Eli Lilly and Co, Vertex and Vifor; receiving grants from Keryx; and receiving research funding from the Department of Veterans Affairs Health Services Research & Development outside the submitted work. Dr. Virani has received honoraria from the American College of Cardiology in his role as the Associate Editor for Innovations, acc.org. The remaining authors declare that they have no relevant financial interests.
Other Disclosures: Dr. Gregg serves as an Editorial Fellow for the Journal of the American Society of Nephrology.
Disclaimer: The interpretation and reporting of these data are the responsibility of the authors and in no way should be viewed as official policy or interpretation of the Department of Veterans Affairs or the US government. The opinions expressed reflect those of the authors and not necessarily those of the Department of Veterans Affairs, NIH or the US government.
References
- 1.Davies MJ, D'Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. Dec 2018;41(12):2669–2701. doi: 10.2337/dci18-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. Nov 26 2015;373(22):2117–28. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 3.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. Aug 17 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 4.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. Jun 13 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes Diabetes Work G. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. Oct 2020;98(4S):S1–S115. doi: 10.1016/j.kint.2020.06.019 [DOI] [PubMed] [Google Scholar]
- 6.Al Rifai M, Mahtta D, Ramsey DJ, et al. Correlates of SGLT-2-inhibitiors use among patients with atherosclerotic cardiovascular disease and type 2 diabetes mellitus: Insights from the department of veterans affairs. Am Heart J. Dec 27 2021;doi: 10.1016/j.ahj.2021.12.006 [DOI] [PubMed] [Google Scholar]
- 7.Nelson AJ, Ardissino M, Haynes K, et al. Gaps in Evidence-Based Therapy Use in Insured Patients in the United States With Type 2 Diabetes Mellitus and Atherosclerotic Cardiovascular Disease. J Am Heart Assoc. Jan 19 2021;10(2):e016835. doi: 10.1161/JAHA.120.016835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold SV, de Lemos JA, Rosenson RS, et al. Use of Guideline-Recommended Risk Reduction Strategies Among Patients With Diabetes and Atherosclerotic Cardiovascular Disease. Circulation. Aug 13 2019;140(7):618–620. doi: 10.1161/CIRCULATIONAHA.119.041730 [DOI] [PubMed] [Google Scholar]
- 9.Maitra NS, Mahtta D, Navaneethan S, et al. A Mistake Not to Be Repeated: What Can We Learn from the Underutilization of Statin Therapy for Efficient Dissemination of Cardioprotective Glucose Lowering Agents? Curr Cardiol Rep. Jun 2022;24(6):689–698. doi: 10.1007/s11886-022-01694-5 [DOI] [PubMed] [Google Scholar]
- 10.Lamprea-Montealegre JA, Madden E, Tummalapalli SL, et al. Association of Race and Ethnicity With Prescription of SGLT2 Inhibitors and GLP1 Receptor Agonists Among Patients With Type 2 Diabetes in the Veterans Health Administration System. JAMA. Sep 6 2022;328(9):861–871. doi: 10.1001/jama.2022.13885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. May 5 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. Oct 8 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 13.Kraus BJ, Weir MR, Bakris GL, et al. Characterization and implications of the initial estimated glomerular filtration rate 'dip' upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int. Mar 2021;99(3):750–762. doi: 10.1016/j.kint.2020.10.031 [DOI] [PubMed] [Google Scholar]
- 14.Xie Y, Bowe B, Gibson AK, McGill JB, Maddukuri G, Al-Aly Z. Clinical Implications of Estimated Glomerular Filtration Rate Dip Following Sodium-Glucose Cotransporter-2 Inhibitor Initiation on Cardiovascular and Kidney Outcomes. J Am Heart Assoc. Jun 2021;10(11):e020237. doi: 10.1161/JAHA.120.020237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Boer IH, Khunti K, Sandusky T, et al. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;45(12):3075–3090. doi: 10.2337/dci22-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu CD, Powe NR, McCulloch CE, et al. Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use Among Hypertensive US Adults With Albuminuria. Hypertension. Jan 2021;77(1):94–102. doi: 10.1161/HYPERTENSIONAHA.120.16281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limonte CP, Hall YN, Trikudanathan S, et al. Prevalence of SGLT2i and GLP1RA use among US adults with type 2 diabetes. J Diabetes Complications. Jun 2022;36(6):108204. doi: 10.1016/j.jdiacomp.2022.108204 [DOI] [PubMed] [Google Scholar]
- 18.McCoy IE, Han J, Montez-Rath ME, Chertow GM, Rhee JJ. Patient and Provider Characteristics Associated With Sodium-Glucose Cotransporter 2 Inhibitor Prescription in Patients With Diabetes and Proteinuric Chronic Kidney Disease. Clin Diabetes. Jul 2020;38(3):240–247. doi: 10.2337/cd19-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahtta D, Ramsey DJ, Lee MT, et al. Utilization Rates of SGLT2 Inhibitors and GLP-1 Receptor Agonists and Their Facility-Level Variation Among Patients With Atherosclerotic Cardiovascular Disease and Type 2 Diabetes: Insights From the Department of Veterans Affairs. Diabetes Care. Feb 1 2022;45(2):372–380. doi: 10.2337/dc21-1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue D, Nishi H, Inoue R, Nangaku M. Regional Distribution of Cardiologists and Prescription Patterns of Sodium-Glucose Transporter-2 Inhibitors in Japan. Int Heart J. 2021;62(3):592–600. doi: 10.1536/ihj.20-716 [DOI] [PubMed] [Google Scholar]