Abstract
Background
Sinonasal adenosquamous carcinoma is rare, and there are almost no studies detailing morphology or characterizing their genetic driver events. Further, many authors have termed sinonasal tumors with combined squamous carcinoma and glands as mucoepidermoid carcinoma but none have analyzed for the presence of MAML2 rearrangement.
Methods
Cases from 2014 to 2020 were collected and diagnosed using World Health Organization criteria. They were tested for p16 expression by immunohistochemistry (70% cut-off), DEK::AFF2 fusion by fluorescence in situ hybridization (FISH) and AFF2 immunohistochemistry, MAML2 rearrangement by FISH, and low- and high-risk HPV by RNA ISH and reverse transcription PCR, respectively. Detailed morphology and clinical features were reviewed.
Results
There were 7 male (64%) and 4 female (36%) patients with a median age of 69 years, most Caucasian (10 of 11 or 91%). Most had tobacco exposure (8/11, 73%) and most presented with epistaxis, a visible nasal mass, and/or facial pain. Several had a precursor papillomas (3 of 11, 27%). The squamous component had variable keratinization, 5 of 11 (46%) of which would be described as keratinizing, 3 non-keratinizing, and 2 with mixed features. All had gland formation, by definition, and 2 of 11 (18%) had ciliated tumor cells. None of the 11 cases had MAML2 rearrangement and one had DEK::AFF2 fusion with associated positive nuclear AFF2 protein immunostaining. Most were p16 positive (7 of 11, 64%) and all 7 of these were hrHPV positive either by RNA ISH or RT-PCR. Two of the p16-negative tumors were positive for lrHPV by RNA ISH. Treatment included surgery alone (4 of 11, 36%), surgery with adjuvant radiation (5 of 11, 45%), and surgery with radiation and chemotherapy (2 of 11, 18%). Four of 11 patients (36%) suffered disease recurrence, two requiring re-operation and who were disease free at last follow-up, one receiving additional chemotherapy and who was alive with disease. The other elected to undergo palliative therapy and died of disease.
Conclusion
Sinonasal adenosquamous carcinoma is a somewhat heterogeneous tumor not infrequently arising ex papilloma and having various drivers including high- and low-risk HPV and rarely DEK::AFF2 fusion. The prognosis appears favorable when proper treatment is possible.
Keywords: Nasal Cavity, Adenosquamous Carcinoma, Paranasal Sinus, DEK::AFF2, Low-risk HPV, High-risk HPV, MAML2
Introduction
Adenosquamous carcinoma (AdSCC) of the sinonasal tract is a rare entity with less than 100 reported cases in the English literature [1–5]. Sinonasal tumors with combined squamous carcinoma and gland formation have been regarded by some authors to represent mucoepidermoid carcinomas (MEC) arising from sinonasal minor salivary gland tissues, and some have even considered tumors like this arising in a background of sinonasal papilloma (“ex papilloma”) as MEC. [6, 7]. However, based on surface mucosal involvement, overt squamous differentiation (rather than just being squamoid or epidermoid), and the immunohistochemical phenotype of the tumors, it is more generally thought that AdSCC is a subtype of SCC [8]. The World Health Organization (WHO) specifically defines AdSCC [9] as a tumor with a biphasic morphology containing both squamous and adenocarcinomatous components that are distinct but in close proximity to each other [10]. While there is some morphologic overlap with MEC, AdSCC is typically associated with dysplasia of the surface epithelium and consistently demonstrates keratinizing squamous differentiation [11], which is rare in MEC. Additionally, most MECs have been shown to harbor a tumor-type specific translocation fusion gene CRTC1::MAML2[12, 13]. Clinically, AdSCC behaves more aggressively than both MEC and conventional (keratinizing) SCC[1, 2, 4, 14, 15]. AdSCC, across all head and neck anatomic subsites, has a 3-year survival rate of 52% and a median survival time of 39 months with up to 80% of patients developing nodal and/or distant metastases [1, 5]. In contrast, metastasis and death from MEC are relatively uncommon [16]. Due to the rarity of sinonasal AdSCC, there are almost no studies detailing morphology or characterizing genetic driver events, such as transcriptionally active human papillomavirus (HPV) [17, 18] or DEK::AFF2 fusion [19–22]. Further, despite the relatively frequent confusion between sinonasal AdSCC and MEC, no studies have examined for the presence of MAML2 rearrangement in these patients. In this study, we examined 11 cases of sinonasal adenosquamous carcinoma, characterized their morphology, and evaluated for key driver events.
Materials and Methods
A natural language search of surgical pathology reports for specimens examined at the Vanderbilt University Medical Center from 2014 to 2020 was performed to identify cases diagnosed as sinonasal AdSCC. Cases were examined microscopically and confirmed as AdSCC using WHO diagnostic criteria [9, 10]. After gathering the patients from Vanderbilt University Medical Center, three cases from collaborators at the University of Miami and the Ohio State University were acquired (from 2021 to 2022). The diagnosis required the presence of distinct areas of invasive SCC intermixed with nests with punched-out (smooth) luminal spaces, with or without intraluminal mucin. Some tumors had goblet cells with intracytoplasmic mucin. As supported by prior studies and the WHO criteria [9], there was no lower percentage cut-off for the amount of glandular or squamous differentiation. If a papilloma component was present, it was diagnosed using WHO criteria, as well. Detailed morphologic characterization of each case was performed, and clinical and demographic data were collected for all patients. The fraction of surface area occupied by glands and/or goblet cells relative to SCC was semiquantitated in 5% increments. Mitotic activity was semiquantitated as very low (almost none detectable), low (occasional mitotic figures), medium (mitotic figures easily found), high (mitotic figures in almost every field), and very high (multiple mitotic figures in every field).
p16 Immunohistochemistry
Immunohistochemistry was performed for p16 on formalin-fixed, paraffin-embedded tissue sections. For the in-house cases, testing utilized the E6H4 antibody (prediluted; Ventana Medical Systems, Inc.) on a Leica Bond automated instrument (Leica Biosystems, Inc.) with antigen retrieval consisting of 10 min in the ER1 proprietary antigen retrieval solution. Primary antibody solution was diluted using Leica’s BOND primary antibody diluent. The Bond Polymer Refine detection system was used for visualization. Slides were then dehydrated, cleared, and coverslipped. Staining was interpreted by one-study pathologist (JSL), using the CAP recommendation [23] defined in oropharyngeal SCC for p16 as a surrogate of high-risk HPV (positive = nuclear and cytoplasmic positivity in > 70% of tumor cells of at least moderate to strong intensity). For the 3 outside cases, p16 immunohistochemistry was performed and interpreted in routine clinical practice at the respective institution using the same interpretation criteria.
HPV Testing
For high-risk HPV, RT-PCR was performed using RNA extracted from two 10-μm unstained slides which were selected for tumor containing areas from the corresponding H&E slides [24]. Testing was performed for E6 and E7 mRNA transcripts of the most common 13 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 52, 56, 58, 59, 66, and 68) [24]. RNA Extraction was performed with Qiagen miRNeasy FFPE kit (QIAGEN Sciences Inc., Gaithersburg, MD) and resulted in between 0.5 and 5 µg of RNA, depending on tumor size. All oligo primers in the assays were purchased from Sigma-Aldrich (St Louis, MO, USA). The reverse transcription reaction was done with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The RT-PCR mixture contained 150 ng of RNA and was incubated at 25 °C for 20 min, 37 °C for 60 min and then 85 °C for 5 min. qPCR was then performed to quantify the cDNA product using Power SYBR Green PCR Master Mix (Applied Biosystems) and 500-nM HPV-type specific primers. Each HPV assay for E6 or E7 from 13 HPV types was individually performed in a separate well on a 384-well PCR plate. The PCR protocol was 95 °C for 10 min and then 36 cycles of amplification (95 °C for 10 s, 58 °C for 15 s, and 60 °C for 15 s). GAPDH and β-actin were included as expression reference controls for qPCR data normalization (normalized to the average expression of these two control genes). Quantitation was expressed as normalized threshold PCR cycle number [24].
For the in-house cases, low-risk HPV in situ hybridization for E6/E7 mRNA was performed by Propath Laboratories (Dallas, TX) using the RNAscope® 2.5 HD—BROWN Manual Assay (Advanced Cell Diagnostics, Inc., Hayward, CA) targeting HPV-associated RNA in the nucleus and cytoplasm of the target cells. Tissue samples that previously stained positive for low-risk HPV, as well as some that were negative, were used as batch control tissues and reacted appropriately. Probes cover HPV types 6, 11, 42, 43, and 44. Cases were read by a single-study pathologist (JSL) and classified in a binary manner as either positive or negative. For one of the outside cases, lrHPV RNA in situ hybridization had been performed as part of clinical practice, and for three of the outside cases, hrHPV RNA in situ hybridization had been performed as part of clinical practice.
DEKFluorescence In Situ Hybridization (FISH) and AFF2 Immunohistochemistry
DEK FISH was performed using a break-apart probe (catalog no.: CT-PAC347; CytoTest, Rockville, MD, USA) as previously described [20]. The result was considered positive with more than 20% of nuclei showing break-apart signals in 50 non-overlapping tumor cells. AFF2 immunohistochemistry was performed using an anti-AFF2 antibody (HPA003139, dilution 1:100, Sigma-Aldrich, St. Louis, MO, USA) as previously described [22]. The result was considered positive with nuclear expression of any intensity in more than 30% of the tumor cells.
Results
In total, 8 cases from the Vanderbilt University Medical Center were confirmed as AdSCC from our original database search. An additional 3 more recently diagnosed cases from collaborators were also identified for a total of 11 cases. All 11 cases examined were from surgical resection specimens. The clinical and pathologic features for all patients are shown in Table 1. There were 7 (64%) males and 4 (36%) females ranging in age from 50 to 74 (mean, 63.8, median 69.0). Primary cross-sectional imaging was available for 10 of 11 patients with 9 (82%) having an identifiable mass. The most common primary location for tumors was the nasal septum, 5 of 11 cases (45%), but tumors also arose in the maxillary sinus (2 of 11, 18%), ethmoid sinus (1 of 11, 9%), and nasal dorsum (1 of 11, 9%). In one patient, a nasal cavity tumor extended to involve the right medial canthus (1 of 9, 11%).
Table 1.
Clinical Features
| Clinical Features | |
|---|---|
| Demographics | |
| Male | 7 (64%) |
| Female | 4 (36%) |
| Age (years and SD) | 64 ± 9 |
| Ethnicity | |
| Caucasian | 10 (91%) |
| African American | 1 (9%) |
| Alcohol Exposure | 4/11 (36%) |
| Smoke Exposure | 8/11 (73%) |
| Locations | |
| Nasal Septum | 5/11 (45%) |
| Maxillary Sinus | 2/11 (18%) |
| Ethmoid Sinus | 1/11 (9%) |
| Inner Canthus (Eye) | 1/11 (9%) |
| Nasal Vestibule | 1/11 (9%) |
| Nasal Dorsum | 1/11 (9%) |
SD Standard deviation
Tobacco use was common, with 8 of 11 patients (73%) endorsing frequent exposure. Alcohol use was less, with only 4 of 11 patients (36%) reporting consumption. Patients were treated either with surgery alone (4 of 11, 36%), surgery plus postoperative radiation (5 of 11, 45%), or surgery plus postoperative radiation and chemotherapy (2 of 11, 18%). The patients were followed for an average of 13.97 months (range 0.5–28) with 4 of 11 patients (36%) experiencing a recurrence and only 1 of those 4 patients dying of disease (10 months postoperatively).
The histopathologic features for all patients are shown in Table 2. The most common features were those essentially necessary for the diagnosis of AdSCC, namely goblet cells (11/11, 100%), mucin (11/11, 100%), tubuloglandular formation (10/11, 91%), and involvement of surface epithelium (10/11, 91%) (Figs. 1 and 2). Other less common features were fibrosis/desmoplasia (10/11, 91%) and keratin pearl formation (4/11, 36%). The adenocarcinoma/gland-forming component varied significantly in extent across the different tumors (range 5–70%) but was, by surface area, the minor one in most cases (average 35%). The squamous component was conventional (keratinizing) in 5 of 11 cases (45%), non-keratinizing in 3 of 11 cases (27%), and mixed areas of both keratinizing (Fig. 1) and non-keratinizing in 2 of 11 cases (18%) (Fig. 3). Intercellular bridges could only be identified within the squamous component in 5 of 11 cases (45%) and only 1 of 11 (9%) cases showed a pattern of cellular discohesion. Interestingly, within the adenocarcinomatous portion of our tumors, cilia were identified in 2 out of 11 cases (18%) (Fig. 4). A precursor lesion was identified in 3 of 11 cases (27%) with 2 of 3 (66%) showing inverted sinonasal papillomas and 1 of 3 (33%) showing exophytic papilloma (Fig. 2).
Table 2.
Histopathologic Features
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mucosal surface involvement | Y | Y | Y | Y | N | Y | Y (focal) | Y | Y | Y | Y |
| Keratin pearls | Y (focal) | Y (focal) | N | N | Y (focal) | Y (multifocal) | N | N | N | N | N |
| Intercellular bridges | N | N | N | N | N | Y (focal) | Y (focal) | Y (focal) | Y (focal) | Y (focal) | N |
| Cellular discohesion | Y (focal) | N | N | N | N | N | N | N | N | N | N |
| Maturing squamous differentiation | Y (focal) | Y (multifocal) | N | N | Y (focal) | Y (multifocal) | N | Y (focal) | Y (focal) | Y (focal) | N |
| Non-keratinizing features | Y | N | Y (focal) | Yes (extensive) | Y | N | N | N | N | N | Y |
| Perineural invasion | N | N | N | N | Y (multifocal) | N | N | N | N | N | Y (multifocal) |
| Vascular invasion | N | N | N | N | N | N | N | N | N | N | N |
| Tubuloglandular formation | Y (focal) | Y (extensive) | N | Y (multifocal) | Y (multifocal) | Y (multifocal) | Y (multifocal) | Y (multifocal) | Y (extensive) | Y (extensive) | Y (multifocal) |
| Mucin | Y (focal) | Y (multifocal) | Y (extensive) | Y (multifocal) | Y (focal) | Y (multifocal) | Y (multifocal) | Y (multifocal) | Y (multifocal) | Y (multifocal) | Y (multifocal) |
| Goblet cells | Y (focal) | Y (focal) | Y (extensive) | Y (focal) | Y (focal) | Y (multifocal) | Y (multifocal) | Y (multifocal) | Y (multifocal) | Y (multifocal) | Y (multifocal) |
| Cilia | Y (focal) | N | N | N | N | N | N | Y (focal) | N | N | N |
| Fraction Surface Area Consisting of Glands and/or Goblet Cells (%) | 5 | 40 | 20 | 30 | 40 | 15 | 50 | 30 | 60 | 70 | 30 |
| Pre-existing papilloma | N | N | Y (inverted) | N | Y (inverted) | N | N | Y (exophytic) | N | N | N |
| Nuclear pleomorphism | Mild | Moderate, focally marked | Mild | Marked diffuse | Mild to moderate | marked (focal) | moderate focal | moderate | Mild | Mild | Marked diffuse |
| Dystrophic calcification | N | N | N | N | N | N | Y (focal) | N | N | N | N |
| Necrosis (% surface area) | N | N | N | multifocal (15%) | N | N | N | N | N | N | N |
| Fibrosis/desmoplasia | N | Y (focal) | Y (extensive) | Y (focal) | Y (multifocal) | Y (multifocal) | Y (focal) | Y (multifocal) | Y (multifocal) | Y (extensive) | Y (multifocal) |
| Nuclear shape | Round | Round | Round/grooved | Round/oval | Round/oval | Round | Round | Round/oval/angulated | Round | Round/oval | Round/oval |
| Mitotic activity | Very Low | Medium | Low | High | High | High | High | Very Low | Medium | High | Very High |
Y Yes, N No, N/A Not Available
Fig. 1.
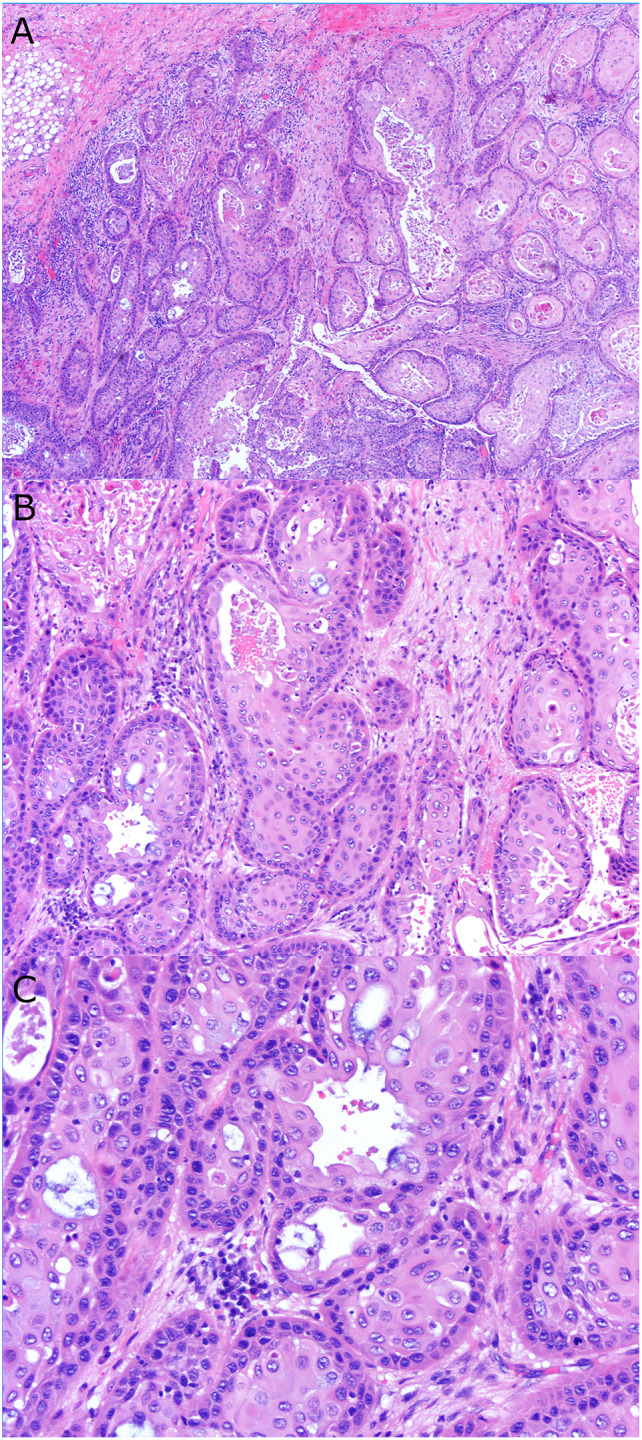
Typical sinonasal AdSCC (Case 6) with keratinizing-type SCC component consisting of A variably sized nests of solid and cystic tumor invading the submucosa. B Solid nests of keratinizing SCC with smaller peripheral basal cells and central keratinized cells with abundant, eosinophilic cytoplasm, mixed with foci of gland formation with smooth luminal borders and mucocytes. C High power showing the moderate nuclear pleomorphism of the tumor cells and scattered mucocytes, mitotic figures, and apoptotic bodies
Fig. 2.
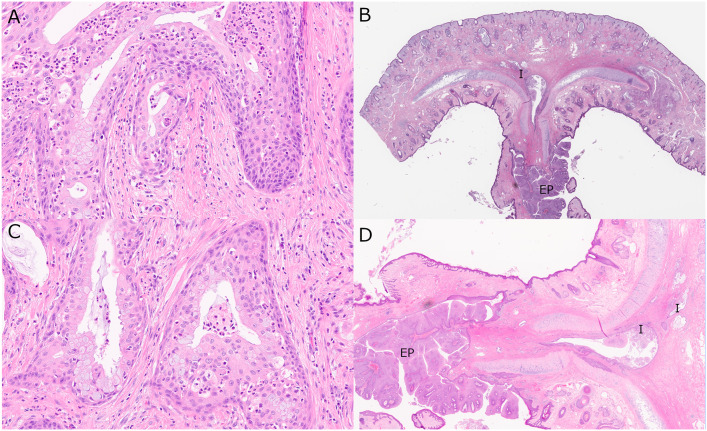
Exophytic papilloma-associated AdSCC (Case 8) showing A an invasive biphasic tumor composed of cuboidal surface cells, some with intracytoplasmic mucin, and with underlying immature squamous epithelium with cells with eosinophilic cytoplasm. B Invasive tumor emerging from the base of a nasal septal exophytic papilloma (I = invasive; EP = papilloma). C On higher power, the mucus cells are more obvious and an infiltrate of neutrophils is present within the epithelium. D High power shows the exophytic papilloma with a few nests of invasive AdSCC with dilated, mucin-filled lumina invading anteriorly into the septum and nasal vestibule
Fig. 3.
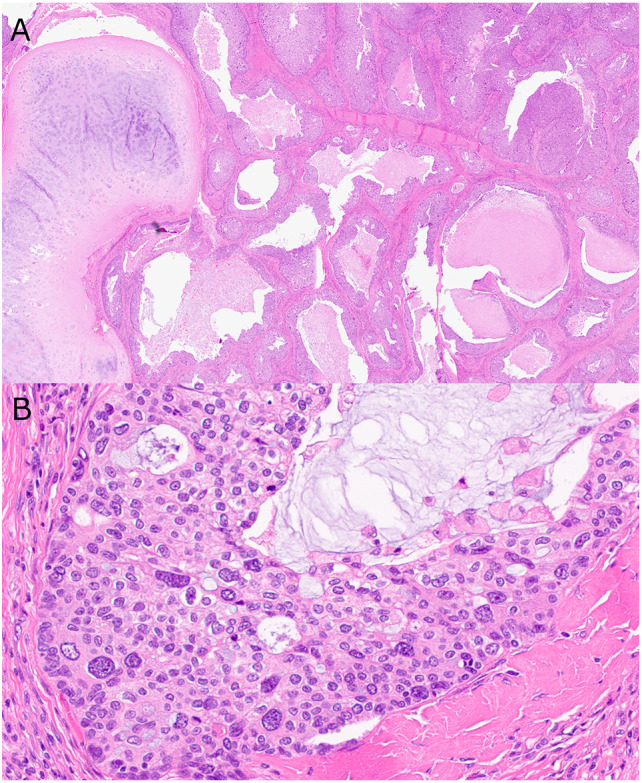
AdSCC (Case 4) showing A variably sized partial solid and partially cystic glands with intraluminal mucin invading the stroma and around septal cartilage and B non-keratinizing SCC with marked nuclear pleomorphism
Fig. 4.
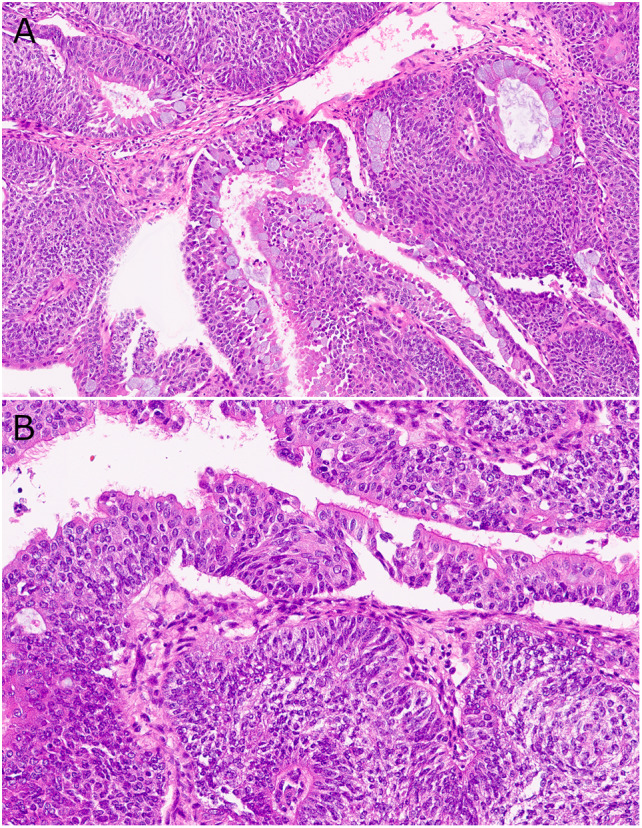
DEK::AFF2 fusion-associated AdSCC (Case 1) showing A a mixture of columnar glandular cells on the surface and around glands with mucus cells and underlying non-keratinizing SCC. B Surface columnar cells with terminal bars and cilia. Note the relatively bland cytologic appearance of the tumor
Tumor cells showed marked nuclear pleomorphism in 4 of 11 cases (36%) (Fig. 3), ranging from focal to diffuse, with 3 of 11 (27%) showing moderate nuclear pleomorphism, and 4 cases (36%) showing only mild pleomorphism. The nuclear shape was typically round (5/11, 45%) or round to ovoid (4/11, 36%). Dystrophic calcification and necrosis were only seen in 1 case (9%) each. Mitotic activity varied between tumors with 1 of 11 cases (9%) demonstrating very high, 5 of 11 cases (45%) demonstrating high, 1 of 11 (9%) demonstrating medium, 1 of 11 (9%) demonstrating low, and 2 of 11 (18%) demonstrating very low mitotic activity (Table 3).
Table 3.
Additional pathologic features
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T stage | N/A | pT2 | pT4a | pT3 | N/A | pT4b | N/A | pT4a | pT4a | pT3 | pT1 |
| N stage | N/A | pNX | pN1 | pN0 | N/A | pN0 | N/A | pNx | pNx | pN0 | pN0 |
| Mucicarmine | N/A | N/A | N/A | N/A | Positive | N/A | N/A | N/A | N/A | N/A | Positive |
| p40 | N/A | N/A | N/A | Positive | N/A | N/A | N/A | N/A | Positive | Positive | Positive |
| p63 | Positive | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Positive | N/A | Positive |
| Cytokeratin AE1/AE3 | Positive | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Ki67 | 15–20% | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Precursor lesion | No | No | Inverted papilloma | No | Inverted papilloma | No | No | Exophytic papilloma | No | No | No |
The molecular and genetic testing for all patients is shown in Table 4. FISH testing for MAML2 gene rearrangement showed all 9 cases tested to be negative (0%). p16 immunohistochemical staining was positive in 7 of 11 cases (64%) using the 70% nuclear and cytoplasmic cut-off (Fig. 5). RNA in situ hybridization for high-risk HPV was positive in the 2 patients tested and reverse transcription PCR for high-risk HPV was positive in 7 of the 9 cases tested, so that, overall, 7 of 11 cases (all of which were also p16 positive) were transcriptionally active high-risk HPV-associated. An additional 2 patients were positive for transcriptionally active low-risk HPV by RNA in situ hybridization. Finally, 1 case (11%) was positive for DEK::AFF2 gene fusion by FISH, which was confirmed by AFF2 immunohistochemistry showing extensive nuclear staining (Fig. 5).
Table 4.
Genetic Drivers
| Case | MAML2 FISH | DEK::AFF2 FISH/IHC | p16 IHC | lrHPV mRNA | hrHPV mRNA RT-PCR | hrHPV mRNA ISH |
|---|---|---|---|---|---|---|
| 1 | − | + | − | + | − | NP |
| 2 | − | − | + | − | + | NP |
| 3 | − | − | − | − | − | NP |
| 4 | − | − | + | − | + | NP |
| 5 | − | − | + | − | + | NP |
| 6 | − | − | + | − | + | NP |
| 7 | − | − | − | − | − | NP |
| 8 | − | − | − | + | − | NP |
| 9 | NP | NP | + | NP | NP | + |
| 10 | NP | NP | + | NP | NP | + |
| 11 | − | − | + | − | NP | + |
FISH fluorescence in situ hybridization, NP not performed, IHC immunohistochemistry, RT-PCR Reverse transcription polymerase chain reaction, lrHPV = low-risk human papillomavirus, hrHPV high-risk human papillomavirus
Fig. 5.
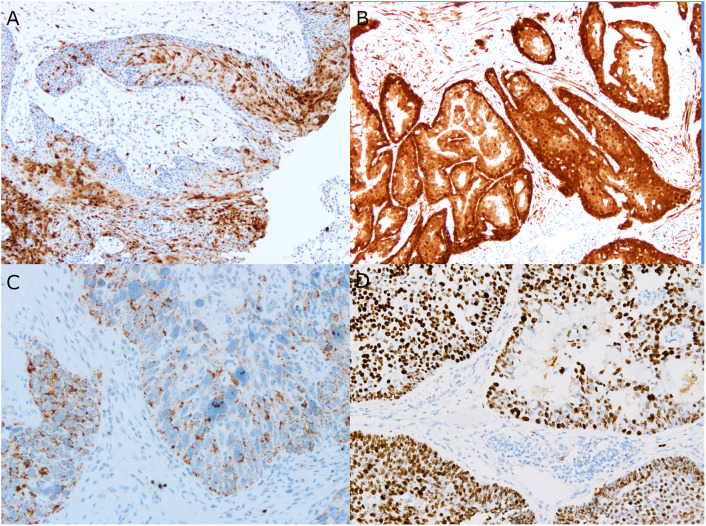
Immunohistochemical and in situ hybridization studies showing positive cases for each of the genetic drivers or surrogate markers. A A high-risk HPV-independent AdSCC showing only patchy nuclear and cytoplasmic expression of p16 (below the 70% cut-off). B A high-risk HPV-associated AdSCC with strong, diffuse nuclear and cytoplasmic p16 expression. C A low-risk HPV-associated AdSCC showing punctate, granular brown nuclear and cytoplasmic staining by RNA in situ hybridization. D AFF2 immunohistochemistry on the single DEK::AFF2 fusion-positive AdSCC showing strong nuclear brown staining in almost all tumor cells and including many of the tumor cells forming glands
Discussion
The current study is one of very few to detail the morphology and to characterize genetic driver events in sinonasal AdSCC. While there is some overlap in morphology between this entity and MEC, there are key differences morphologically and, as shown in this study, in the genetic driver events.
Sinonasal AdSCC occurs primarily in the 6th decade of life on average with no particular sex predilection. The nasal septum is the most commonly affected site which may be secondary to the location containing respiratory epithelium more readily exposed to low- and high-risk HPV from pharyngeal reflux. The tumors are somewhat heterogeneous in that they vary substantially in the amount of gland formation, the degree of mucus cell differentiation, and in the amount of mitotic activity and degree of nuclear atypia. Some are relatively bland, with minimal nuclear pleomorphism, little mitotic activity (cases 1, 3, 9, 10), pushing borders, and even rarely with tumor forming cilia. Others are markedly infiltrative with marked nuclear pleomorphism and mitotic activity (cases 2, 4, 6, 11). Some arise ex-sinonasal papilloma. The heterogeneity is reflected in their molecular features, with most tumors driven by transcriptionally active high-risk HPV, a few by low-risk HPV, and even occasional cases by DEK::AFF2 fusion [19, 20, 22]. In all, we found a genetic “driver” in 10 of the 11 cases (90.9%).
The distinction between sinonasal tract AdSCC and MEC is somewhat difficult and has been a source of confusion in head and neck pathology [9] [2]. Many cases have been reported as sinonasal tract MEC, and some even reported as arising ex papilloma [6]. Sinonasal tract MECs have been reported as case reports and one larger series, by Wolfish et al. [7], of 19 cases from the Armed Forces Registry of Pathology (AFIP) and author consultation files. They reported that these represented 0.075% of all sinonasal tumors over the time period analyzed, giving an idea of their rarity [7]. The key distinguishing morphologic features between AdSCC and MEC are surface squamous dysplasia/involvement and maturing squamous differentiation, both of which, when present, favor AdSCC. While Wolfish et al. reported that they excluded tumors with keratinizing squamous differentiation, 6 of their cases did have surface involvement which was reportedly difficult to tell as “arising from the surface” versus “involving the surface.” They did not have any molecular testing. In our study, we found all 9 tested cases of AdSCC to be negative for MAML2 rearrangement. This is supported by the data of Kass et al. [2], who analyzed AdSCC across the head and neck region for MAML2 by FISH and found all 7 of their sinonasal tract cases to be negative for rearrangement. It seems that, given the rarity of what has been described as sinonasal tract MEC, one could be much more conclusive for that diagnosis by having positive rearrangement by MAML2 FISH testing.
The prognosis of AdSCC has been found, in general, to be worse than that for conventional SCC across head and neck anatomic subsites [5, 15]. This was supported more specifically in the sinonasal tract by Vazquez et al. [4], who analyzed disease outcomes for sinonasal SCC and subtypes from the Surveillance, Epidemiology, and End Results (SEER) database. They found that of all the subtypes, AdSCC had the worst prognosis. There was no central review for tumor classification and no accounting for HPV status; however, so it is unclear how many of these 31 tumors really were strictly defined AdSCC, nor how many of these rare tumors were HPV-associated compared to the remaining patients. In our study, the prognosis was actually quite favorable. This may not be a surprise as most patients had high- or low-risk HPV-associated tumors, arose from an existing sinonasal papilloma, or had DEK::AFF2 fusion [19, 20, 22] and bland morphology, all of which have been associated with more favorable survival compared to conventional sinonasal SCC[17, 25–27].
Overall, our findings in AdSCC match well with sinonasal tract SCC in general, with a predilection for the nasal cavity, frequent smoking association, a significant fraction of high-risk HPV, and rare cases associated with sinonasal papillomas, low-risk HPV, or DEK::AFF2 fusion [17, 25]. This small series, somewhat opposed to prior data on head and neck AdSCC, found quite favorable prognosis, probably due to the high fraction of high-risk HPV-associated patients.
Conclusion
In summary, this series of sinonasal AdSCC helps define the morphology and genetic drivers of tumor growth. These drivers are similar to conventional sinonasal SCC and support that it is considered a histologic subtype of SCC, although it does appear that AdSCC is more frequently HPV associated, either high or low risk. The presence of established driver events that match conventional SCC along with the lack of MAML2 translocation in any of our cases argues strongly that these tumors are not a type of salivary gland tumor.
Acknowledgements
The authors would like to thank the staff of the Tissue Pathology Shared Resource (TPSR) for the excellent work on the immunostains used in this study.
Author Contributions
All authors whose names appear on the submission made substantial contributions to the conception or design of the work, to the acquisition, analysis, or interpretation of data, and/or drafted the work or revised it critically for important intellectual content including approving the version to be published. They agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Research was performed using discretionary funds from the Department of Pathology, Microbiology, and Immunology. The work also utilized the Translational Pathology Shared Resource (TPSR) at Vanderbilt University Medical Center which is supported by the NCI/NIH Cancer Center Support Grant 5P30 CA68485-19 and the Shared Instrumentation Grant S10 OD023475.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest as it relates to this work.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was performed with approval of the institutional review boards of Vanderbilt University Medical Center and complies with required ethical standards. Given the retrospective nature of the study, with consultation with the institutional review boards, it was determined that the study did not need ethical approval. Patients were never contacted, and we did not require informed consent or specific consent to publish.
Consent to Participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schick U, Pusztaszeri M, Betz M, Ghadjar P, Demiroz C, Kaanders JH, et al. Adenosquamous carcinoma of the head and neck: report of 20 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(3):313–320. doi: 10.1016/j.oooo.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Kass JI, Lee SC, Abberbock S, Seethala RR, Duvvuri U. Adenosquamous carcinoma of the head and neck: molecular analysis using CRTC-MAML FISH and survival comparison with paired conventional squamous cell carcinoma. Laryngoscope. 2015;125(11):E371–E376. doi: 10.1002/lary.25519. [DOI] [PubMed] [Google Scholar]
- 3.Masand RP, El-Mofty SK, Ma XJ, Luo Y, Flanagan JJ, Lewis JS., Jr Adenosquamous carcinoma of the head and neck: relationship to human papillomavirus and review of the literature. Head Neck Pathol. 2011;5(2):108–116. doi: 10.1007/s12105-011-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vazquez A, Khan MN, Blake DM, Patel TD, Baredes S, Eloy JA. Sinonasal squamous cell carcinoma and the prognostic implications of its histologic variants: a population-based study. Int Forum Allergy Rhinol. 2015;5(1):85–91. doi: 10.1002/alr.21418. [DOI] [PubMed] [Google Scholar]
- 5.Lee RJ, Lin T, Lee SA, Lee KK, Christensen RE. Importance of tumor extent in adenosquamous carcinoma of the head and neck: a retrospective cohort study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(2):114–120. doi: 10.1016/j.oooo.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Nudell J, Chiosea S, Thompson LD. Carcinoma Ex-Schneiderian Papilloma (Malignant Transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2014;8(3):269–286. doi: 10.1007/s12105-014-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfish EB, Nelson BL, Thompson LD. Sinonasal tract mucoepidermoid carcinoma: a clinicopathologic and immunophenotypic study of 19 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2012;6(2):191–207. doi: 10.1007/s12105-011-0320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alos L, Castillo M, Nadal A, Caballero M, Mallofre C, Palacin A, et al. Adenosquamous carcinoma of the head and neck: criteria for diagnosis in a study of 12 cases. Histopathology. 2004;44(6):570–579. doi: 10.1111/j.1365-2559.2004.01881.x. [DOI] [PubMed] [Google Scholar]
- 9.Prasad ML, Wenig BM. WHO classification of Tumours Editorial Board - Head and Neck Tumours [Internet; beta version ahead of print] 5. Lyon: IARC Press; 2022. Adenosquamous carcinoma. [Google Scholar]
- 10.Cardesa A, Zidar N, Alos L. Adenosquamous carcinoma. In: Barnes L, Eveson J, Reichart P, Sidransky D, editors. Pathology and Genetics Head and Neck Tumours. Lyon: IARC Press; 2005. pp. 130–131. [Google Scholar]
- 11.Fonseca FP, Ramos LM, Vargas PA, de Almeida OP, Lopes MA, Santos-Silva AR. Oral adenosquamous carcinoma: evidence that it arises from the surface mucosal epithelium. Histopathology. 2012;61(2):321–323. doi: 10.1111/j.1365-2559.2012.04257.x. [DOI] [PubMed] [Google Scholar]
- 12.Chiosea SI, Dacic S, Nikiforova MN, Seethala RR. Prospective testing of mucoepidermoid carcinoma for the MAML2 translocation: clinical implications. Laryngoscope. 2012;122(8):1690–1694. doi: 10.1002/lary.22419. [DOI] [PubMed] [Google Scholar]
- 13.Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34(8):1106–1121. doi: 10.1097/PAS.0b013e3181de3021. [DOI] [PubMed] [Google Scholar]
- 14.Fiacchini G, Benettini G, Trico D, Torregrossa L, Vianini M, Picariello M, et al. Human papillomavirus-related head and neck adenosquamous carcinoma: a systematic review and individual patient data meta-analysis. Oral Oncol. 2021;119:105252. doi: 10.1016/j.oraloncology.2021.105252. [DOI] [PubMed] [Google Scholar]
- 15.Mehrad M, Trinkaus K, Lewis JS. Jr. Adenosquamous Carcinoma of the Head and Neck: a case-control study with conventional squamous cell carcinoma. Head Neck Pathol. 2016;10(4):486–493. doi: 10.1007/s12105-016-0727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen MM, Roman SA, Sosa JA, Judson BL. Histologic grade as prognostic indicator for mucoepidermoid carcinoma: a population-level analysis of 2400 patients. Head Neck. 2014;36(2):158–163. doi: 10.1002/hed.23256. [DOI] [PubMed] [Google Scholar]
- 17.Bishop JA, Guo TW, Smith DF, Wang H, Ogawa T, Pai SI, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37(2):185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop JA, Westra WH. Human papillomavirus-related carcinomas of the sinonasal tract. Mod Pathol. 2012;25(Supplement 2):305A. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rooper LM, Agaimy A, Dickson BC, Dueber JC, Eberhart CG, Gagan J, et al. DEK-AFF2 Carcinoma of the Sinonasal Region and Skull Base: detailed clinicopathologic characterization of a distinctive entity. Am J Surg Pathol. 2021;45(12):1682–1693. doi: 10.1097/PAS.0000000000001741. [DOI] [PubMed] [Google Scholar]
- 20.Kuo YJ, Lewis JS, Jr, Zhai C, Chen YA, Chernock RD, Hsieh MS, et al. DEK-AFF2 fusion-associated papillary squamous cell carcinoma of the sinonasal tract: clinicopathologic characterization of seven cases with deceptively bland morphology. Mod Pathol. 2021;34(10):1820–1830. doi: 10.1038/s41379-021-00846-2. [DOI] [PubMed] [Google Scholar]
- 21.Bishop JA, Gagan J, Paterson C, McLellan D, Sandison A. Nonkeratinizing squamous cell carcinoma of the Sinonasal Tract with DEK-AFF2: further solidifying an emerging entity. Am J Surg Pathol. 2021;45(5):718–720. doi: 10.1097/PAS.0000000000001596. [DOI] [PubMed] [Google Scholar]
- 22.Kuo YJ, Lewis JS, Jr, Truong T, Yeh YC, Chernock RD, Zhai C, et al. Nuclear expression of AFF2 C-terminus is a sensitive and specific ancillary marker for DEK::AFF2 carcinoma of the sinonasal tract. Mod Pathol. 2022;35(11):1587–1595. doi: 10.1038/s41379-022-01117-4. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JS, Jr, Beadle B, Bishop JA, Chernock RD, Colasacco C, Lacchetti C, et al. Human papillomavirus testing in Head and Neck Carcinomas: Guideline from the College of American Pathologists. Arch Pathol Lab Med. 2018;142(5):559–597. doi: 10.5858/arpa.2017-0286-CP. [DOI] [PubMed] [Google Scholar]
- 24.Gao G, Chernock RD, Gay HA, Thorstad WL, Zhang TR, Wang H, et al. A novel RT-PCR method for quantification of human papillomavirus transcripts in archived tissues and its application in oropharyngeal cancer prognosis. Int J Cancer. 2012;132(4):882–890. doi: 10.1002/ijc.27739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elgart K, Faden DL. Sinonasal squamous cell carcinoma: etiology, pathogenesis, and the role of human papilloma virus. Curr Otorhinolaryngol Rep. 2020;8(2):111–119. doi: 10.1007/s40136-020-00279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma A, Tang AL, Takiar V, Wise-Draper TM, Langevin SM. Human papillomavirus and survival of sinonasal squamous cell Carcinoma Patients: a systematic review and Meta-analysis. Cancers (Basel) 2021;13(15):3677. doi: 10.3390/cancers13153677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen ES, Risbud A, Birkenbeuel JL, Murphy LS, Goshtasbi K, Pang JC, et al. Prognostic factors and outcomes of De Novo Sinonasal squamous cell carcinoma: a systematic review and Meta-analysis. Otolaryngol Head Neck Surg. 2022;166(3):434–443. doi: 10.1177/01945998211021023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.