Abstract
Introduction
Postoperative hemorrhage after adult cranial neurosurgery is a serious complication with substantial morbidity and mortality.
Research question
We investigated if an extended preoperative screening and an early treatment of previously undetected coagulopathies may decrease the risk of postoperative hemorrhage.
Methods
A prospective study cohort of patients undergoing elective cranial surgery and receiving the extended coagulatory work-up were compared to a propensity matched historical control cohort. The extended work-up included a standardized questionnaire on the patient's bleeding history as well as coagulatory tests of Factor XIII, von-Willebrand-Factor and PFA-100®. Deficiencies were substituted perioperatively. The primary outcome was determined as the surgical revision rate due to postoperative hemorrhage.
Results
The study cohort and the control cohort included 197 cases each, without any significant difference in the preoperative intake of anticoagulant medication (p = .546). Most common interventions were resections of malignant tumors (41%), benign tumors (27%) and neurovascular surgeries (9%) in both cohorts. Imaging revealed postoperative hemorrhage in 7 cases (3.6%) in the study cohort and 18 cases (9.1%) in the control cohort (p = .023). Of these, revision surgeries were significantly more common in the control cohort with 14 cases (9.1%) compared to 5 cases (2.5%) in the study cohort (p = .034). Differences in mean intraoperative blood loss were not significant with 528 ml in the study cohort and 486 ml in the control cohort (p = .376).
Conclusion
Preoperative extended coagulatory screening may allow for revealing previously undiagnosed coagulopathies with subsequent preoperative substitution and thereby reduction of risk for postoperative hemorrhage in adult cranial neurosurgery.
Keywords: Extended coagulation, Cranial surgery, Postoperative hemorrhage, Complication, Coagulation substitution
Highlights
-
•
Patients undergoing cranial surgery often harbor unknown coagulation disorders.
-
•
Extended coagulation screening allowed perioperative substitution of factors.
-
•
A cohort with extended screening was compared to a historical cohort without.
-
•
The perioperative coagulatory optimization reduced postoperative hemorrhage rates.
Abbreviations
- ASA
acetylsalicylic acid
- FXIII
Factor XIII
- IQR
interquartile range
- NOAC
new oral anticoagulants
- PFA
Platelet-Function-Analyzer
- PH
postoperative hemorrhage
- PT
prothrombin time
- PTT
partial thromboplastin time
- SD
standard deviation
- suppl
supplementary
- TC
thrombocyte count
- vWF
von-Willebrand factor
1. Introduction
The management of surgical complications continues to gain relevance in modern neurosurgery. A postoperative hemorrhage (PH) at the surgical site represents one of the most common and at the same time serious postoperative adverse events (AE), typically ranging between 0.8% and 5.9% in recent investigations; although some studies find far higher rates of up to 40.4% with analyses confounded by factors such as concomitant medication, diseases and most predominantly the varying definitions of PH (Fukamachi et al., 1985; Greuter et al., 2019; Kalfas and Little, 1988; Seifman et al., 2011; Touho et al., 1986; Wilhelmy et al., 2020). Prior to any discourse, it is of utmost importance to indicate a common definition to delineate de novo hemorrhages found on routine postoperative imaging from PH accompanied by clinical deterioration and requiring surgical revision – the latter of which being the uniformly accepted descriptor of principal academic and clinical interest.
There is arguably no better management of AEs than to prevent them. For the prevention of PH in particular, one may reason that the preoperative detection of coagulopathies allows for their preemptive adequate substitution by transfusion of blood components or specific drugs, such as Factor XIII (Seifman et al., 2011). In consideration of the coagulatory system's complex physiology, ordinary preoperative laboratory diagnostics may fail to identify yet unknown coagulopathies in patients, which may manifest during or after an invasive procedure such as neurosurgery for the first time. The value of an extended preoperative coagulatory screening of parameters to reduce the occurrence of PH is still unknown due to the shortage of works investigating this matter (Kalfas and Little, 1988; Seifman et al., 2011).
This study was conceived to investigate the occurrence rate of PH after extended coagulatory work-up and substitution in a consecutive cohort of patients undergoing elective cranial surgery at our institution.
2. Patients & methods
2.1. Patient selection
Beginning in December 2018, we implemented extended coagulatory work-up for all patients undergoing any elective intracranial procedure, any patient aged over 18 years thus qualified for analysis. The procedures included resections of benign and malign intracranial neoplasms, neurovascular surgery, cranioplasties, shunt implantations, evacuation of hematomas and biopsies. Patients undergoing emergency procedures were excluded.
This prospectively enrolled study cohort was propensity matched to a historical control cohort in a 1:1 fashion according to type of intervention as per the nationwide standardized operational code (OPS), sex and lastly age of the subjects. Extended coagulatory work-up generally was not conducted for the control cohort.
2.2. Coagulatory diagnostics and substitution
The extended coagulatory diagnostics were conceived in cooperation between the senior authors of neurosurgery (MW, BM) and hemostaseology (MH, CW) with reference to a past study by Koscielny et al. (2004) It encompassed a standardized 12-item questionnaire (Table 2) and coagulatory tests of Factor XIII (FXIII), von-Willebrand-Factor (vWF) and Platelet-Function-Analyzer-100® (PFA) generally sampled 48 h before the procedure. The vWF analysis encompassed both activity and antigen parameters, likewise, the PFA included both adenosindiphosphate (ADP) and epinephrin parameters. Any positively answered item on the questionnaire prompted laboratory diagnostics beyond the aforementioned extended work-up (i.e. Fibrinogen, serum levels of Factors II, VIII, IX and X) and evaluation of possible therapeutic strategies after consultation with hemostaseologists. Standard parameters consisting of the prothrombin time (PT), partial thromboplastin time (PTT) and thrombocyte count (TC) as per standard of practice were sampled for both the extended diagnostics cohort and the control cohort.
Table 2.
Preoperative questionnaire for the screening of bleeding history. Any positively answered item would lead to further specific coagulation testing beyond the extended diagnostics described in this study.
| Yes | No | |
|---|---|---|
| □ | □ | 1. Have you noticed an increased occurrence of nosebleeds recently? |
| □ | □ | 2. Have you noticed the occurrence of “bruises” or small, punctiform bleeding without obvious trauma? |
| If you answered “yes” to this question, please state whether these symptoms also occurred on the torso or in other areas that are unusual for you! | ||
| □ | □ | 3. Have you noticed frequent bleeding from the gums? |
| □ | □ | 4. Do you bleed or bruise more than once or twice a week? |
| □ | □ | 5. Do you have the impression that cuts or abrasions (e.g. shaving) tend to bleed longer? |
| □ | □ | 6. Have you ever had prolonged or increased bleeding after or during any surgery (e.g. tonsillectomy, appendectomy, birth)? |
| □ | □ | 7. Did you have prolonged and increased bleeding during or after dental extractions? |
| □ | □ | 8. Have you ever been given blood products after or during surgery? |
| □ | □ | 9. Does any relative have a history of bleeding tendencies? |
| □ | □ | If you are female: 10. Do you have the impression that your menstruation is lengthened (>7 days) and/or increased? |
| □ | □ | 11. Do you take painkillers or antirheumatic medication? If so, please enter the name of the medication! |
| □ | □ | 12. Are you taking any other medication? If so, please enter the name of the medication! |
For any intracranial surgery, antithrombotic or anticoagulatory medication was generally stopped without substitute, if possible, after consultation with in-house cardiologists, angiologists or other pertinent specialists of internal medicine or surgery, depending on diagnoses requiring such medication. The intake of antithrombotic medication (e.g. acetylsalicylic acid, clopidogrel) was paused seven days prior to surgery and new oral anticoagulants (NOACs; e.g. rivaroxaban, apixaban, dabigatran) were paused 48 h prior to surgery. Coumarin derivatives (e.g. phenprocoumon) were paused and antagonized with Vitamin K agonists or substitution of a prothrombin complex containing Factors II, VII, IX and X as well as Protein C and Protein S until normalization of the PT and PTT were achieved. Antithrombotics and NOACs were further paused for seven to fourteen days after surgery. Postoperative analyses were limited to PT, PTT and TC, performed at 48 h after surgery, except for cases with proven deficiency in any extended diagnostics parameter, which would then also be monitored postoperatively.
Deficiencies in any parameter, either in common (PT, PTT, TC) or extended diagnostics (FXIII, vWF, PFA, Fibrinogen, Factors II, VII, VIII, X), were substituted perioperatively according to recommendations by senior hemostaseologists and pertinent literature. Supplementary Table 1 lists products used for substitution of the respective deficiency. Products were administered in a timely manner prior to surgery adapted to body weight, and the respective parameter controlled after a repeat blood draw to screen for any remaining deficiency.
2.3. Outcome analyses
The data were recorded prospectively and analyzed retrospectively. The primary outcome was predetermined as the surgical revision rate due to PH. A revision surgery was performed for patients who postoperatively developed new neurological impairment such as sensorimotor deficits, seizures or reduced alertness attributable to hemorrhage found on postoperative imaging. The revision procedure was then carried out as rapidly as feasible in the individual case. PH without any clear clinical correlate which were found incidentally on postoperative imaging and did not require revision surgery were additionally recorded and incorporated in the secondary analyses.
Further secondary analyses encompassed intergroup testing of demographic data, types of procedures, surgical times, intraoperative blood loss volume, frequency and amount of blood component substitution, rates of thromboembolic complications as well as economic analysis of additive expenses for the extended diagnostics. An assessment of intraoperatively increased hemorrhage was drawn from surgical reports.
Statistical analysis was conducted with IBM SPSS software in its 26th edition, the level of significance was defined a priori as α = 0.05. Intergroup testing was conducted by Chi-square tests and Fisher's exact tests for non-parametric variables. Student's t-test and the Welch test were used for testing of statistical differences between means of continuous variables.
3. Results
3.1. Demographic data
The extended diagnostics cohort included 188 patients undergoing 197 surgeries between December 2018 and June 2019. The control cohort included 197 patients with 197 surgeries between April 2016 and June 2018. Table 1 lists demographic data, proportions of surgeries and preoperative intake of anticoagulatory or antithrombotic medication for both cohorts. There were no statistical differences between the distribution of any of the parameters included in the matching algorithm. Every listed procedure was conducted via craniotomy except for stereotactic biopsies.
Table 1.
Demographic data at baseline and types of surgeries conducted for both cohorts. Others include evacuation of intracranial abscesses, deep tissue infections and reconstructions of anterior skull base defects. ∗Stereotactic biopsy without craniotomy. ASA – ayetylsalicylic acid; NOAC – new oral anticoagulants; P – level of significance. ∗Stereotactic biopsy without craniotomy. ASA – ayetylsalicylic acid; NOAC – new oral anticoagulants; P – level of significance.
| Study Cohort (N = 197) | Control Cohort (N = 197) | P | |
|---|---|---|---|
| Age (years) | |||
| Mean | 57 | 57 | .840 |
| Median | 57 | 56 | |
| Standard deviation | 16 | 14 | |
| Range | 19–97 | 19–84 | |
| Variance | 267 | 193 | |
| Female sex, n (%) | 101 (51) | 99 (52) | .920 |
| Surgery, n (%) | |||
| Benign tumor resection | 52 (26) | 53 (27) | .997 |
| Malign tumor resection | 80 (41) | 81 (41) | |
| Neurovascular surgery | 18 (9) | 18 (9) | |
| Hematoma evacuation | 2 (1) | 2 (1) | |
| Cranioplasty | 23 (12) | 24 (12) | |
| Shunt implantation | 6 (3) | 6 (3) | |
| Stereotactic biopsy∗ | 9 (5) | 9 (5) | |
| Others | 7 (4) | 4 (2) | |
| Preoperative Medication | |||
| ASA (%) | 25 (13) | 21 (11) | .556 |
| ASA + Clopidogrel (%) | 1 (0,5) | – | |
| ASA + NOAC (%) | 2 (1) | 1 (0.5) | |
| Clopidogrel (%) | – | – | |
| NOAC (%) | 8 (4) | 2 (1) | |
| Marcumar (%) | 1 (0.5) | 1 (0.5) | |
3.2. Coagulation diagnostics
When comparing preoperative coagulation testing between study and control cohorts, there were significant differences of both preoperative PT (study cohort median 118% [IQR 110–120] vs. control cohort median 107% [IQR 101–113]; p < .001) and PTT (study cohort median 29 s [IQR 27–31] vs. control cohort median 28 s (Palmer et al., 1994; Samii and Matthies, 1997; Seifman et al., 2011; Taylor et al., 1995; Touho et al., 1986; Wilhelmy et al., 2020); p = .018) values, although all values were in their respective normal reference ranges (PT 70–120%; PTT 26–37 s). No significant difference between means of preoperative TC was found (study cohort 253 G/l [SD 83] vs. control cohort 264 G/l [SD 77]; p = .203) and both were within the reference range of 150–450 G/l.
The postoperative values, examined 48 h after surgery, demonstrated significant differences between cohorts but were all within reference range as well: PT: 105% (IQR 98–114) vs. 97% (IQR 90–103; p < .001), PTT 30 s (IQR 28–32) vs. 28 s (IQR 26–31; p < .001), TC 210 G/l (SD 68) vs. 223 G/l (SD 78; p = .085).
For the study cohort, the median preoperative FXIII value amounted to 107% (IQR 85–121; reference range 70–140), the median vWF activity value amounted to 144% (IQR 121–181; reference range [depending on blood type] 52–200), the median vWF antigen value was 148% (IQR 112–212; reference range [depending on blood type] 52–200), the median PFA ADP value was 84 s (IQR 73–97; reference range 68–121) and the median PFA epinephrin value was 111 s (IQR 93–134; reference range 84–160).
3.3. Outcome analyses
The mean surgical time differed significantly between study and control cohorts: 147 min (SD 81) vs. 166 min (SD 90; p = .031). The mean volume of intraoperative blood loss was found to be without statistical difference with 528 ml (study cohort; SD 508) vs. 486 ml (control cohort; SD 436; p = .376).
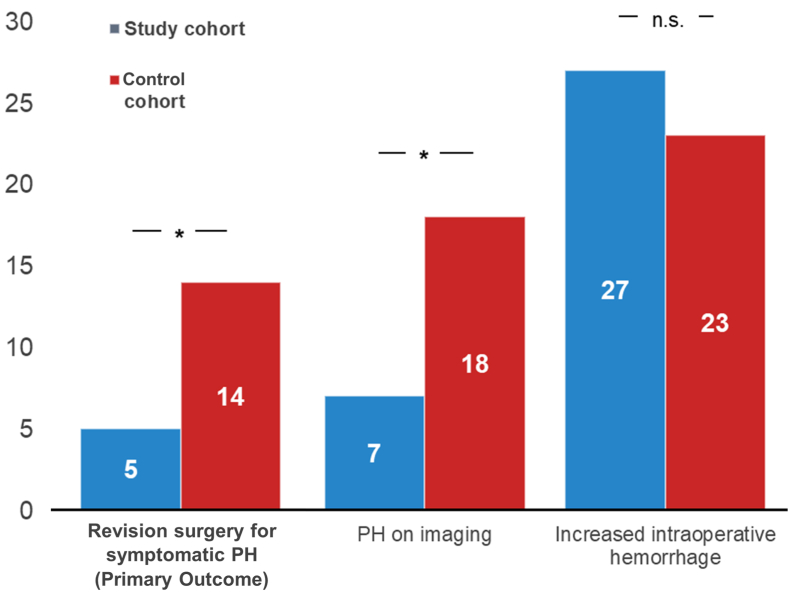
Fig. 1 depicts primary outcome data. While no statistically significant difference concerning intraoperative blood loss was found, the control cohort exhibited a significantly higher rate of PH on imaging (18 [9.1%] vs. 7 [3.6%]; p = .023) and revision surgery due to PH (14 [7.1] vs. 5 [2.5]; p = .034).
Fig. 1.
Figure 1 Graph bars of revision surgeries due to PH (the primary outcome), PH on postoperative imaging and intraoperative increased blood loss. Asterisks denote significant differences in intergroup comparisons at the .05 level; n.s. – non-significant.
Half of the patients suffering from PH that required revision surgery in the control cohort did not exhibit any addition disability in the course after revision, recovering without sequelae, while the other half suffered from some degree of neurological disability (suppl. Table 2). In the study cohort, 3 patients (60%) underwent revision surgery for PH without any hematoma-associated disability on follow up, while one patient (20%) suffered from major disability in an apallic state. Of the 5 patients undergoing revision surgery for PH in the study cohort, one patient (20%) died within 30 days of hospitalization for unrelated reasons (suppl. Table 2).
With few exceptions, all revision surgeries were timed 2–5 h after diagnosis of the PH. The majority of PH were situated intraparenchymally in both the study (n = 3; 60%) and control cohort (n = 10; 71.4%), 3 (40%) were epidural in the study cohort and 3 (21.4%) in the control cohort, and 1 one was subdural (7.1%) in the control cohort.
3.4. Substitution of blood products and adverse events
In the study cohort, blood products were substituted for 70 surgeries (35.5%) perioperatively compared to 43 surgeries (21.8%) in the control cohort (p = .003).
The types of blood products transfused are listed in Table 3. Compared to the control cohort, FXIII, fibrinogen, tranexamic acid and platelets were substituted significantly more common in the perioperative setting. Fibrinogen and FXIII were commonly substituted jointly, as were vWF and Factor VIII. Calcium gluconate was substituted significantly more often in the control cohort.
Table 3.
Number of products substituted perioperatively for both cohorts. P – level of significance. ∗combined in the product Haemate ®.
| Product | Study cohort, n (%) | Control cohort, n (%) | P |
|---|---|---|---|
| FXIII | 22 (11.1) | – | – |
| Fibrinogen | 22 (11.1) | 7 (3.5) | .048 |
| vWF∗ | 17 (8.7) | – | – |
| Faktor VIII∗ | 17 (8.7) | – | – |
| Tranexamic acid | 43 (21.8) | 16 (8.1) | .020 |
| Platelet transfusion | 24 (12.2) | 4 (2) | .038 |
| Prothrombin complex | 5 (2.5) | 2 (1.0) | .449 |
| Calcium gluconate | 8 (4.1) | 22 (11.2) | .012 |
| Fresh frozen plasma | 1 (0.5) | 5 (2.5) | .215 |
| Desmopressin | 10 (5.1) | 10 (5.1) | 1.000 |
There were no significant differences in the occurrence of adverse events related to substitution or transfusion of products (Table 4). One patient (0.5%) in the study cohort complained of vertigo and palpitations during transfusion of a platelet bag, which was removed immediately. This patient suffered no symptomatic PH or PH on imaging. No instances of transfusion-associated transmission of pathogens were noted.
Table 4.
Number of adverse events stratified by cohort. P – level of significance. ∗e.g. hepatitis B, C, human immune-deficiency virus, etc. ±one case of possible allergic reaction during platelet transfusion, causing vertigo and palpitations without cardiovascular depression.
| Study Cohort |
Control Cohort |
P | |
|---|---|---|---|
| N (%) | N (%) | ||
| Thromboembolic | 8 (4.1) | 6 (3.0) | .586 |
| Transfusion-associated infection∗ | 0 (−) | 0 (−) | – |
| Allergic reaction± | 1 (0.5) | 0 (−) | .317 |
3.5. Past bleeding history questionnaire
The preoperative questionnaire was completed for 147 of 197 surgeries (74.6%) in the study cohort. Thirty-six patients undergoing 36 surgeries (18.3%) answered at least one of the items 1–10 on the preoperative questionnaire with “yes”. Item 1 was the most common in 8 of these 36 patients (22.2%), followed by item 8 (n = 7; 19.4%) and item 2 (n = 5; 13.9%; see Table 2). Outcome analyses stratified by past bleeding history are demonstrated in Table 5. In total, there were no statistically significant differences in the occurrences of hemorrhage events between subgroups.
Table 5.
Outcome analyses stratified by past bleeding history. ∗Data missing for 50 patients.
| Past bleeding History Questionnaire∗ |
P | ||
|---|---|---|---|
| Positive (N = 36) | Negative (N = 111) | ||
| Increased intraoperative hemorrhage, N (%) | 7 (19) | 15 (14) | ,423 |
| PH on imaging, N (%) | 3 (8) | 4 (4) | ,363 |
| Revision for PH, N (%) | 3 (8) | 2 (2) | ,095 |
3.6. Cost analysis
Expenses for the extended coagulatory diagnostics amounted to a cumulative 13 124.10 EUR (136.71 USD) for the study cohort, and a cumulative 285.65 EUR (314.67 USD) for routine parameters in each cohort. These data translated to 68.07 EUR (74.99 USD) per surgery in the study cohort and 1.45 EUR (1.60 USD) per surgery in the control cohort. To mitigate the occurrence of one PH needing revision surgery would therefore require expenses of 1458.23 EUR (1606.39 USD).
4. Discussion
Our study was conceived to investigate the benefit of extended coagulation diagnostics for neurosurgical patients undergoing elective intracranial surgery. We implemented routine testing of FXIII, vWF and PFA in addition to a self-constructed questionnaire to screen for any occult coagulation deficiencies in the perioperative setting. Any deficiencies were compensated for by transfusion of blood products perioperatively, and in doing so, we managed to reduce the occurrence rate of a symptomatic PH needing revision surgery by 64.3% compared to a control cohort without the extended work-up. This substantial mitigation of a potentially fatal or disabling postoperative complication, however, comes with a minor risk for adverse events and an exponential increase in costs.
The sequelae of a PH cannot be overstated in severity, particularly since this specific surgical complication may occur comparatively often. A number of publications puts the incidence between 0.8% and 40.4%, a wide range stemming from heterogenous definitions (Chughtai et al., 2019; Haber et al., 2019; Kalfas and Little, 1988; Lassen et al., 2011; Touho et al., 1986). Variable authors have settled for the PH that necessitates surgical revision and evacuation as common ground for analysis between studies, which we adopted for our primary outcome (Bullock et al., 1990; Chan et al., 1989; Gerlach et al., 2003; Palmer et al., 1994; Seifman et al., 2011; Zetterling and Ronne-Engström, 2004).
Functional outcome for neurosurgical patients suffering from complications is chiefly dependent on the type of surgery, the underlying pathology and the patient's baseline status. While the typical acute hallmark of a PH is an altered level of consciousness within the first 6–48 h after surgery, long term outcomes after a PH are difficult to predict and are surprisingly scarce in published literature (Dickinson et al., 1998; Harders et al., 1985; Taylor et al., 1995). In a study by Li et al., patients receiving acetylsalicylic acid (ASA) prior to an intracranial surgery were prone to suffer PH and subsequently decreased functional autonomy at 6 months compared to controls (Li et al., 2013). Kalfas et al. report neurological deterioration in 80% of their 40 patients with PH in relation to their expected outcome after the index surgery (Kalfas and Little, 1988). Lassen et al. report a hematoma-related mortality rate of 22% in their retrospective analysis of 2630 patients (Lassen et al., 2011). In our study, 42.1% of patients in both cohorts requiring revision surgery for PH suffered from significant neurological decline as an immediate consequence of the hematoma, with only partial recovery for some patients, and one patient died in the follow up after revision surgery although the association with the PH seems debatable at least. Our mortality and disability rates seem comparatively low, which may be owed to our policy of monitoring patients in the intensive care/postoperative recovery unit for at least 24 h after craniotomy. However, seeing that only 26.3% of PHs occurred within 24 h and 63.2% within 48 h, an even longer observation period may be recommended (Lassen et al., 2011). While in clinical practice it is customary to revise PHs as quickly as feasible, there is not enough data to conclude a definitive cut-off in terms of time expedited after clinical deterioration until revision surgery. Anecdotally, the patients undergoing revision surgery within 2–3 h experienced the most significant disability in contrast to those being revised after a greater time period, although this notion is largely confounded by the fact that a major disability with corresponding PH prompts a timelier reaction.
Perhaps the most often reported aspect of PH in literature is constituted by factors impacting on the risk of occurrence. The contributors are not limited to medical conditions or surgical technique, Seifman et al. collated numerous studies linking patient positioning, intraoperative hypertension, alcohol consumption, antithrombotic and anticoagulant medication, FXIII deficiencies and intraoperative blood loss to PH risk (Algattas et al., 2016; Benvenuti and Gagliardi, 2004; Bullock et al., 1990; Chan et al., 1989; Chen et al., 2012; Chughtai et al., 2019; Churnin et al., 2015; Dickinson et al., 1998; Merriman et al., 1979; Nakajima et al., 2002; Palmer et al., 1994; Samii and Matthies, 1997; Seifman et al., 2011; Zetterling and Ronne-Engström, 2004). Palmer et al. further identified incompletely resected neoplasms as a potential “beds” for PH due to their hypervascularization and dysfunctional blood-brain barrier, with meningioma resections causing almost double the rate of PH (6.2%) than trauma cases (Palmer et al., 1994). The authors conclude that antiplatelet medication represented the most common contributor to the risk of PH and may be avoided in 75% of cases. FXIII deficiency was prospectively studied by one study group as well, which reported a 6.4-fold increase of the PH risk compared to patients with normal postoperative FXIII levels and therefore recommend preoperative screening (Gerlach et al., 2000, 2002). The FXIII is exacerbated by a concomitant deficiency of fibrinogen (Yan et al., 2018). This notion appears to be especially relevant in neurosurgical patients, due to the depletory effects of craniotomies on postoperative FXIII levels and hence marked risk of PH (Gerlach et al., 2000; Menges et al., 1994).
These observations raise the question of routine preoperative screening and treatment of such avoidable risks, which has not been investigated as much. In fact, some authors advocate against even the routine preoperative assessment of PT and PTT, let alone extended coagulation profiling, in cases of negative bleeding history (Akhunzada et al., 2018; Dützmann et al., 2012). Neurosurgical units adopting this dogma instead rely on standardized questionnaires to screen for possible abnormalities, which has been shown to alleviate economic expenses (Harley et al., 2019). Through implementation of a questionnaire conceived in unison with specialists in hemostaseology, we were not able to demonstrate a significant impact on our primary outcome PH occurrence. Naturally, this is confounded by our approach to routinely conduct extended diagnostics with the most common originators of coagulopathies aside from conventional coagulation panels – despite negative bleeding history – in an academic initiative to elucidate the utility of one over the other. It may be questionable whether the “less is more” philosophy of only relying on questionnaires emerges as more cost effective in the long run. Literature so far lacks any conclusive juxtaposition of costs of a thorough extended coagulation screening and therapeutic strategy against the sparse strategy of only screening by history. Still, one must consider the potential drastic long-term effects of PH on the individual's autonomy and the cumulative health care burden, which altogether seem immeasurable. In light of this, the excessive costs for extended coagulation diagnostics and substitution may very well justify the drastic reduction of PH occurrence, supported by a safe risk profile.
5. Study limitations
The results of this study are based on cohorts matched by a propensity score, which is tailored to certain aspects of the surgery pairs but cannot account for every contributing variable. The differing PH rates may hence be attributable to other confounding factors that changed between the years, such as surgeon experience, intraoperative hemostyptics used and anesthesiological monitoring protocol. In this context, we prioritized matching surgery types within the specified time period in our propensity model, which resulted in a mismatch in patient number (188 vs. 197 patients) with 197 matched surgeries each. This mismatch of 5% may be seen as a confounding factor, but we believe its effect to ultimately be negligible on the primary outcome effect size, particularly considering that none of these surgeries were followed by a PH and they were matched tumor resections exclusively. A randomized multicenter clinical trial enrolling large numbers of patients would deliver significantly clearer results. However, the ethical justification of a randomization to a “non-extended” arm in this case would be very debatable.
Another limitation may stem from the wide array of diverse procedures included in both cohorts. We deliberately chose this approach in favor of recruiting only a specific subset of entities to generate reproducible results from an unselected population. The inclusion of minor procedures with a naturally low risk of PH thus may have led to underestimation of the effect size of our outcome parameters.
6. Conclusion
The routine implementation of extended coagulation diagnostics, including screening for vWF and FXIII deficiencies, and substitution of respective products significantly cut PH rates with a safe risk profile in this propensity matched retrospective comparison. These findings must be considered against the marked economic cost.
Funding
No funding was received for this research.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study group acquired approval by the local ethics committee (Ethikkommission der Technischen Universität München), Registration No. 465/19 S-SR.
Informed consent
For this retrospective study, the requirement for informed consent was waived by the ethics committee (Ethikkommission der Technischen Universität München).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Dr W Peul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bas.2023.101756.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Akhunzada N.Z., Tariq M.B., Khan S.A., Sattar S., Tariq W., Shamim M.S., Dogar S.A. Value of routine preoperative tests for coagulation before elective cranial surgery. Results of an institutional audit and a nationwide survey of neurosurgical centers in Pakistan. World Neurosurg. 2018;116:e252–e257. doi: 10.1016/j.wneu.2018.04.183. [DOI] [PubMed] [Google Scholar]
- Algattas H., Kimmell K.T., Vates G.E. Risk of reoperation for hemorrhage in patients after craniotomy. World Neurosurg. 2016;87:531–539. doi: 10.1016/j.wneu.2015.09.020. [DOI] [PubMed] [Google Scholar]
- Benvenuti L., Gagliardi R. Risk of postoperative hemorrhage after intracranial surgery after early nadroparin administration: results of a prospective study. Neurosurgery. 2004;55:1229–1230. doi: 10.1227/01.neu.0000144047.17046.cd. author reply 1230-1221. [DOI] [PubMed] [Google Scholar]
- Bullock R., Hanemann C.O., Murray L., Teasdale G.M. Recurrent hematomas following craniotomy for traumatic intracranial mass. J. Neurosurg. 1990;72:9–14. doi: 10.3171/jns.1990.72.1.0009. [DOI] [PubMed] [Google Scholar]
- Chan K.H., Mann K.S., Chan T.K. The significance of thrombocytopenia in the development of postoperative intracranial hematoma. J. Neurosurg. 1989;71:38–41. doi: 10.3171/jns.1989.71.1.0038. [DOI] [PubMed] [Google Scholar]
- Chen C.C., Hsu P.W., Lee S.T., Chang C.N., Wei K.C., Wu C.T., Hsu Y.H., Lin T.K., Lee S.C., Huang Y.C. Brain surgery in patients with liver cirrhosis. J. Neurosurg. 2012;117:348–353. doi: 10.3171/2012.4.jns111338. [DOI] [PubMed] [Google Scholar]
- Chughtai K.A., Nemer O.P., Kessler A.T., Bhatt A.A. Post-operative complications of craniotomy and craniectomy. Emerg. Radiol. 2019;26:99–107. doi: 10.1007/s10140-018-1647-2. [DOI] [PubMed] [Google Scholar]
- Churnin I., Shakal A., Seifi A. Gender association with postoperative hemorrhage patient safety indicator in the United States from 2000 to 2012. J. Crit. Care. 2015;30:1124–1125. doi: 10.1016/j.jcrc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Dickinson L.D., Miller L.D., Patel C.P., Gupta S.K. Enoxaparin increases the incidence of postoperative intracranial hemorrhage when initiated preoperatively for deep venous thrombosis prophylaxis in patients with brain tumors. Neurosurgery. 1998;43:1074–1081. doi: 10.1097/00006123-199811000-00039. [DOI] [PubMed] [Google Scholar]
- Dützmann S., Gessler F., Marquardt G., Seifert V., Senft C. On the value of routine prothrombin time screening in elective neurosurgical procedures. Neurosurg. Focus. 2012;33:E9. doi: 10.3171/2012.7.focus12219. [DOI] [PubMed] [Google Scholar]
- Fukamachi A., Koizumi H., Nukui H. Postoperative intracerebral hemorrhages: a survey of computed tomographic findings after 1074 intracranial operations. Surg. Neurol. 1985;23:575–580. doi: 10.1016/0090-3019(85)90006-0. [DOI] [PubMed] [Google Scholar]
- Gerlach R., Raabe A., Zimmermann M., Siegemund A., Seifert V. Factor XIII deficiency and postoperative hemorrhage after neurosurgical procedures. Surg. Neurol. 2000;54:260–264. doi: 10.1016/s0090-3019(00)00308-6. discussion 264-265. [DOI] [PubMed] [Google Scholar]
- Gerlach R., Scheuer T., Beck J., Woszczyk A., Seifert V., Raabe A. Risk of postoperative hemorrhage after intracranial surgery after early nadroparin administration: results of a prospective study. Neurosurgery. 2003;53:1028–1034. doi: 10.1227/01.neu.0000088565.15719.22. discussion 1034-1025. [DOI] [PubMed] [Google Scholar]
- Gerlach R., Tölle F., Raabe A., Zimmermann M., Siegemund A., Seifert V. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke; J. Cerebral Circul. 2002;33:1618–1623. doi: 10.1161/01.str.0000017219.83330.ff. [DOI] [PubMed] [Google Scholar]
- Greuter L., Ullmann M., Mariani L., Guzman R., Soleman J. Effect of preoperative antiplatelet or anticoagulation therapy on hemorrhagic complications in patients with traumatic brain injury undergoing craniotomy or craniectomy. Neurosurg. Focus. 2019;47:E3. doi: 10.3171/2019.8.focus19546. [DOI] [PubMed] [Google Scholar]
- Haber M.A., Abd-El-Barr M., Gormley W., Mukundan S., Sodickson A.D., Potter C.A. Neurosurgical complications: what the radiologist needs to know. Emerg. Radiol. 2019;26:331–340. doi: 10.1007/s10140-019-01672-5. [DOI] [PubMed] [Google Scholar]
- Harders A., Gilsbach J., Weigel K. Supratentorial space occupying lesions following infratentorial surgery early diagnosis and treatment. Acta Neurochir. 1985;74:57–60. doi: 10.1007/bf01413279. [DOI] [PubMed] [Google Scholar]
- Harley B., Abussuud Z., Wickremesekera A., Shivapathasundram G., Rogers N., Buyck H. Preoperative screening for coagulopathy in elective neurosurgical patients in Wellington Regional Hospital and survey of practice across Australia and New Zealand. J. Clin. Neurosci. : Off. J. Neurosurg. Soc. Australasia. 2019;64:201–205. doi: 10.1016/j.jocn.2019.01.048. [DOI] [PubMed] [Google Scholar]
- Kalfas I.H., Little J.R. Postoperative hemorrhage: a survey of 4992 intracranial procedures. Neurosurgery. 1988;23:343–347. doi: 10.1227/00006123-198809000-00010. [DOI] [PubMed] [Google Scholar]
- Koscielny J., Ziemer S., Radtke H., Schmutzler M., Pruss A., Sinha P., Salama A., Kiesewetter H., Latza R. A practical concept for preoperative identification of patients with impaired primary hemostasis. Clinic. Appl. Thromb./Hemost. : Off. J. Int. Acad. Clinic. Appl. Thromb./Hemost. 2004;10:195–204. doi: 10.1177/107602960401000301. [DOI] [PubMed] [Google Scholar]
- Lassen B., Helseth E., Rønning P., Scheie D., Johannesen T.B., Mæhlen J., Langmoen I.A., Meling T.R. Surgical mortality at 30 days and complications leading to recraniotomy in 2630 consecutive craniotomies for intracranial tumors. Neurosurgery. 2011;68:1259–1268. doi: 10.1227/NEU.0b013e31820c0441. discussion 1268-1259. [DOI] [PubMed] [Google Scholar]
- Li X., Sun Z., Zhao W., Zhang J., Chen J., Li Y., Ye Y., Zhao J., Yang X., Xiang Y., Li G., Mao J., Zhang W., Zhang M., Zhang W. Effect of acetylsalicylic acid usage and platelet transfusion on postoperative hemorrhage and activities of daily living in patients with acute intracerebral hemorrhage. J. Neurosurg. 2013;118:94–103. doi: 10.3171/2012.9.jns112286. [DOI] [PubMed] [Google Scholar]
- Menges T., von Lessen A., Welters I., Wagner R.M., Ruwoldt R., Boldt J., Hempelmann G. [Intracranial hemorrhage and hemostasis. Monitoring patients after intracranial hemorrhage by determination and follow-up of activation products of blood coagulation] Infusionstherapie und Transfusionsmedizin. 1994;21:244–250. [PubMed] [Google Scholar]
- Merriman E., Bell W., Long D.M. Surgical postoperative bleeding associated with aspirin ingestion. Report of two cases. J. Neurosurg. 1979;50:682–684. doi: 10.3171/jns.1979.50.5.0682. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Yasui T., Nishikawa M., Kishi H., Kan M. The role of postoperative patient posture in the recurrence of chronic subdural hematoma: a prospective randomized trial. Surg. Neurol. 2002;58:385–387. doi: 10.1016/s0090-3019(02)00921-7. discussion 387. [DOI] [PubMed] [Google Scholar]
- Palmer J.D., Sparrow O.C., Iannotti F. Postoperative hematoma: a 5-year survey and identification of avoidable risk factors. Neurosurgery. 1994;35:1061–1064. doi: 10.1227/00006123-199412000-00007. discussion 1064-1065. [DOI] [PubMed] [Google Scholar]
- Samii M., Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40:11–21. doi: 10.1097/00006123-199701000-00002. discussion 21-13. [DOI] [PubMed] [Google Scholar]
- Seifman M.A., Lewis P.M., Rosenfeld J.V., Hwang P.Y. Postoperative intracranial haemorrhage: a review. Neurosurg. Rev. 2011;34:393–407. doi: 10.1007/s10143-010-0304-3. [DOI] [PubMed] [Google Scholar]
- Taylor W.A., Thomas N.W., Wellings J.A., Bell B.A. Timing of postoperative intracranial hematoma development and implications for the best use of neurosurgical intensive care. J. Neurosurg. 1995;82:48–50. doi: 10.3171/jns.1995.82.1.0048. [DOI] [PubMed] [Google Scholar]
- Touho H., Hirakawa K., Hino A., Karasawa J., Ohno Y. Relationship between abnormalities of coagulation and fibrinolysis and postoperative intracranial hemorrhage in head injury. Neurosurgery. 1986;19:523–531. doi: 10.1227/00006123-198610000-00005. [DOI] [PubMed] [Google Scholar]
- Wilhelmy F., Hantsche A., Wende T., Kasper J., Reuschel V., Frydrychowicz C., Rasche S., Lindner D., Meixensberger J. Perioperative anticoagulation in patients with intracranial meningioma: No increased risk of intracranial hemorrhage? PLoS One. 2020;15 doi: 10.1371/journal.pone.0238387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M.T.S., Rydz N., Goodyear D., Sholzberg M. Acquired factor XIII deficiency: a review. Transfus. Apher. Sci. : Off. J. World Apheresis Assoc. : Off. J. Eur. Soc. Haemaph. 2018;57:724–730. doi: 10.1016/j.transci.2018.10.013. [DOI] [PubMed] [Google Scholar]
- Zetterling M., Ronne-Engström E. High intraoperative blood loss may be a risk factor for postoperative hematoma. J. Neurosurg. Anesthesiol. 2004;16:151–155. doi: 10.1097/00008506-200404000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.