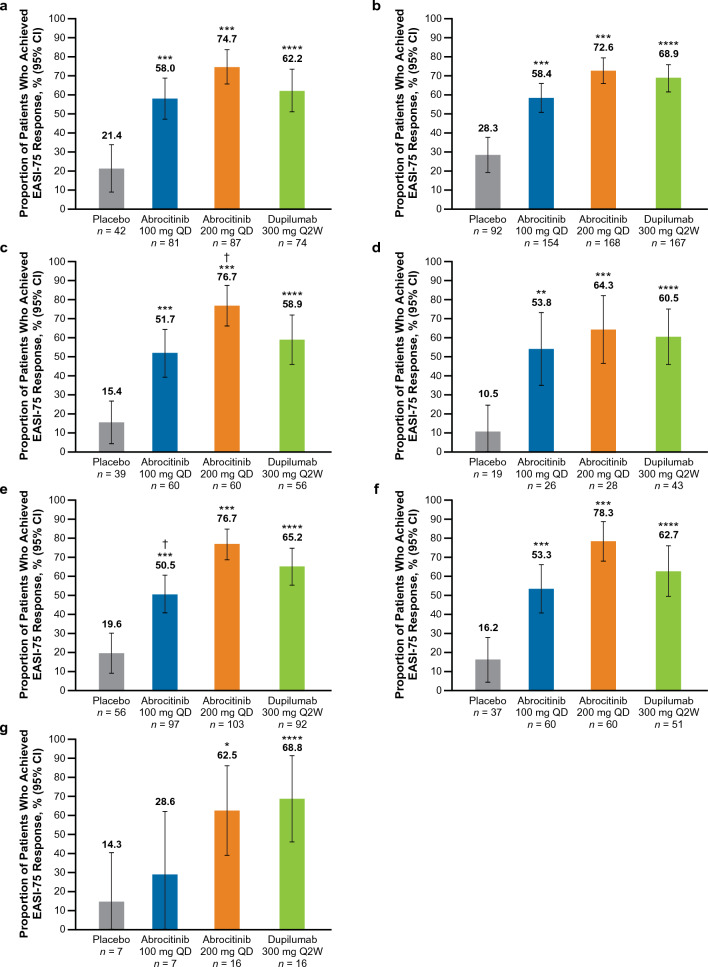
Fig. 2.
%BSA percentage of body surface area, EASI Eczema Area and Severity Index, EASI-75 ≥ 75% improvement from baseline in EASI, IGA Investigator’s Global Assessment, QD once daily, Q2W once every 2 weeks. EASI-75 rates at week 16 in patients with baseline a IGA 4, b EASI > 21, c EASI > 38, d Failure or intolerance to prior systemic agents,a e %BSA > 50, f %BSA > 65 and g in the combined subgroup.b *p < 0.05, **p < 0.01, and ***p < 0.001 for abrocitinib versus placebo. ****Significant difference between dupilumab versus placebo based on 95% CIs for the estimated difference. †p < 0.05 for abrocitinib versus dupilumab. aExcluding patients who took only corticosteroids. bThe combined subgroup was defined as patients with baseline IGA 4, and EASI > 21, and %BSA > 50, and failure or intolerance to prior systemic agents (excluding patients who took only corticosteroids)