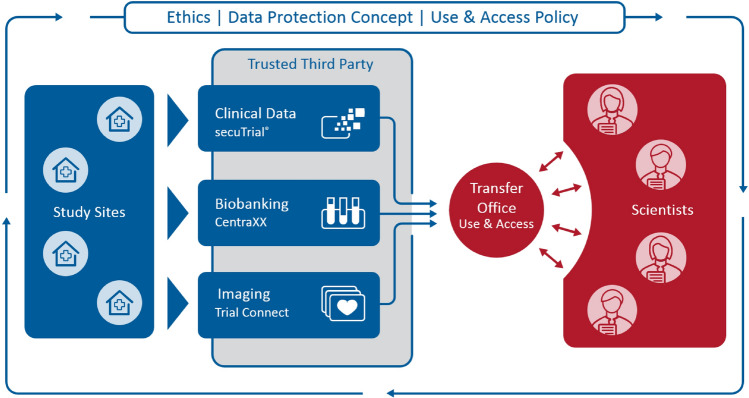
Fig. 2.
DZHK research platform in the context of data acquisition and biological sample collection from patients recruited in DZHK studies (left, blue) and data as well as biological sample secondary use by scientists (right, red). The framework is defined by the Ethics and Data Protection Concepts as well as the DZHK Use and Access Policy. Study sites primarily use the IT systems for data documentation, while scientists interact with the Transfer Office. The Trusted Third Party enables record linkage and ensures proper pseudonymisation and adherence to informed consents