Abstract
This study investigated the differences in health outcomes associated with ferulic acid (FA) supplementation in animals before the induction of diabetes with streptozotocin (STZ) treatment and post-STZ treatment. 18 male Wistar rats were equally distributed into three groups: groups 1 and 2 received FA (50 mg/kg body weight) supplementation one week before STZ treatment (60 mg/kg body weight, intraperitoneal) and one week after STZ treatment, respectively; group 3 received STZ without FA supplementation. FA supplementation was continued for 12 weeks after STZ treatment. The results indicated no difference in glucose and lipid profile with FA supplementation. However, FA supplementation reduced lipid and protein oxidative damage in the heart, liver and pancreas and increased glutathione in the pancreas. The results indicate that while oxidative damages were positively affected by FA, it was not sufficient to improve metabolic markers of diabetes.
Keywords: Ferulic acid, Polyphenols, Diabetes, Antioxidants, Oxidative stress, Streptozotocin
1. Introduction
Millions of people worldwide are affected by diabetes mellitus (DM), which ranks among the most prevalent endocrine disorders. The diabetic condition is characterised by hyperglycaemia which results from either decreased glucose utilisation by the tissue, reduced uptake of glucose by the cells or increased gluconeogenesis in the liver [1]. Chronic hyperglycemia at the cellular level is linked to multiple impairments, resulting in a range of secondary health outcomes collectively called diabetic complications. These complications encompass diabetic retinopathy, diabetic nephropathy, diabetic neuropathy, and diabetic cardiomyopathy [2]. An important hallmark of prolonged hyperglycaemia is oxidative stress. It can also result from increased lipolysis and elevated free fatty acid (FFA) levels in the tissue in type 2 DM [3]. Oxidative stress consequently results in oxidative tissue damage, which can trigger diabetic complications. Impairment of glucose regulation in the human body has various consequences. Increased FFA level in plasma is considered a cause and a result of insulin resistance leading to type 2 DM [4]. The plasma FFA level not only reflects the intake of dietary lipids but is also linked to the balance between de novo fatty acid synthesis, fatty acids storage and lipolysis of triglycerides (TG). There are earlier reports of impairment of this balance in diabetic patients [3]. The multimodal interaction of oxidative stress, inflammation, hyperglycemia and hyperlipidemia is, therefore, responsible for the progression of the disease [2]. Therapeutic interventions for diabetes include a variety of pharmacological agents along with lifestyle changes such as diet and exercise. Natural antioxidants such as polyphenols are gaining traction in research due to their potential therapeutic effect against a wide range of ailments such as diabetes [5], cardiovascular diseases [6], cancers [7] and neurodegenerative diseases [8]. Since polyphenols are abundant in plant-based foods, significant quantities are attained by consuming vegetables, fruits and beverages. Epidemiological studies have reported that in populations with a higher intake of polyphenols or polyphenol-rich food, there is a lower risk of developing metabolic diseases, indicating the preventive effect of polyphenols [9]. These findings are significant in type 2 DM when factoring in global expenditure on diabetes care. However, investigations on the preventive effect of polyphenols in type 2 DM by pre-treatments are rare. Only one study by Hokayem et al. explored the effect of the pre-treatment of polyphenols in humans [10].
The present study investigates the preventive effect of ferulic acid (FA) in type 2 DM by pre-treatment. Ferulic acid (FA) is a ubiquitous polyphenol in the hydroxycinnamic acids group found in large quantities in cereals, cocoa and herbs [11]. Studies have pointed to FA's remarkable bioactive potential in conditions such as diabetes [12,13] and cardiovascular diseases [14,15]. Numerous animal studies have documented FA supplementation's ability to alleviate DM-associated manifestations, including its ability to counteract oxidative damage in tissue, decrease blood glucose and plasma insulin levels, and improve lipid profiles [12,13,[16], [17], [18], [19], [20], [21]]. These animal studies have used an FA dose ranging from 10 to 70 mg/kg body weight [16,[19], [20], [21]]. Previous studies indicate that a dose of 50 mg/kg body weight of FA effectively modulated markers associated with diabetes and reported no differences when doses were increased beyond 50 mg/kg body weight in rats [16,22]. FA-associated systemic effects in these studies were noticeable within seven days of starting the supplementation, and the total duration of the supplementation in most studies varied anywhere between 2 and 10 weeks [16,[19], [20], [21]]. Although the anti-diabetic effect of FA was investigated extensively, no study design, either in humans or in animals, has previously investigated the preventive effects of FA in type 2 DM. Such differences between the preventive and therapeutic effects of FA supplementation in experimental DM models are a critical knowledge gap. Being a potent antioxidant in vivo, we hypothesize that pre-supplementation of FA could help to neutralise the free radicals generated by streptozotocin (STZ) in the pancreas, thereby decreasing STZ toxicity and consequently improving glucose regulation and preventing lipotoxicity associated with type 2 DM. Hence, among individuals predisposed to diabetes, the preventive effect of FA could translate to improved antioxidant status at the tissue level, thereby preventing insulin resistance and lipotoxicity associated with hyperglycaemia. Therefore, to understand the difference between FA supplementation's preventive and therapeutic effect in DM animal models, FA supplementation before induction of DM should be compared with FA supplementation after DM induction in the animals. The present study addresses that gap by studying FA supplementation's ability before STZ treatment and post-STZ treatment to improve disease-related symptoms of type 2 DM. The study also determines whether FA supplementation before or after STZ treatment modulates lipid metabolism and limits lipid and protein oxidative damage related to type 2 DM.
2. Materials and methods
2.1. Materials
All chemicals utilized in the study were procured from Sigma-Aldrich (St. Louis, MO, USA) unless specified otherwise. Streptozotocin was purchased from Merck Millipore (Darmstadt, Germany, Cat. # 572201). Ferulic acid was purchased from Sigma-Aldrich (St. Louis, MO, USA, Cat. # 822070). The kits for clinical biochemistry analysis (Glucose, Cat. # 04657527190; Total cholesterol, Cat. # 04718917190; LDL-cholesterol, Cat. # 07005806190; HDL-cholesterol, Cat. # 05401488190; TG, Cat. # 04657594190) were purchased from Roche Diagnostics (Risch-Rotkreuz, Switzerland). FFA Elisa kit (Cat. # MBS280654) and DAG Elisa kit (Cat. # MBS750727) from MyBiosource Inc. (San Diego, USA). Rat Ins1/Insulin ELISA kit (Cat. # RAB0904) from Sigma-Aldrich (St. Louis, MO, USA). Adult male Wistar rats used in this study were bred in-house at the Animal Research Facility in the College of Medicine and Health Sciences, UAE University.
2.2. Animals and experimental protocol
The experiments followed institutional guidelines in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The study's ethical approval was obtained from the College of Medicine and Health Sciences Animal Research Ethics Committee at UAE University (approval number ERA_2017_5662). 18 male Wistar rats were used in the study. The sample size was calculated using Mead's Resource Equation (E = N–B-T, where E: degree of freedom, N: no. of samples-1, B: blocking component-1, T: treatment groups-1). The animals (mean body weight 262.12 ± 1.65 g and blood glucose 114.84 ± 2.00 mg/dl) were randomly divided by the investigator into three groups, PRE-STZ (n = 6), POST-STZ (n = 6) and STZ (n = 6, control group). No significant differences were observed in the mean body weight and blood glucose levels between the groups (p > 0.05). The animal housing conditions were three animals per cage under controlled conditions of 12-h light and 12-h dark cycle, 50% humidity and 25±3 °C room temperature. The animals were fed a standard pellet diet (Table 1) and were provided water ad libitum throughout the study.
Table 1.
SAFE® A03 feed composition.
| Centesimal Composition* | (g/kg) |
|---|---|
| Cereal | 692 |
| Animal Proteins | 60 |
| Vegetal Proteins | 202 |
| Vitamins and Minerals |
46 |
|
Nutritional Composition |
(%) |
| Nitrogen Free Extract | 52% |
| of which Starch | 33.5% |
| of which Sugars | 4.4% |
| Crude Protein | 21.1% |
| Crude Fat | 5.1% |
| Crude Ash | 5.4% |
| Crude Fibre | 4% |
| Moisture | 12.1% |
*Feed ingredients include wheat, maize, wheat bran, barley, extruded soybeans, soybean meal, hydrolyzed fish proteins, inactivated brewer's yeast, calcium carbonate, pre-mixture of vitamins, pre-mixture of minerals, and dicalcium phosphate.
The treatment protocol was as follows. From day 1 of the study, the PRE-STZ group received FA (50 mg/kg body weight) by oral gavage. On day 8, DM was induced in all the animals. DM animal models were established with a single intraperitoneal injection of STZ (60 mg/kg body weight) in citrate buffer. Bodyweight and random blood glucose (OneTouch Ultra 2, LifeScan) were measured immediately before experiments. Random blood glucose levels established DM in all 18 animals. The FA supplementation in the PRE-STZ group continued throughout the study. On day 15 of the study, FA was supplemented in the POST-STZ group (50 mg/kg body weight) via oral gavage. The third unsupplemented group (STZ) served as the control group in the study. All animals were sacrificed by decapitation at the end of the FA supplementation, 14 weeks in PRE-STZ and 12 weeks in POST-STZ. Blood and organs were harvested for further analysis.
2.3. Biochemical assay
The serum biochemical parameters, including glucose, total cholesterol, LDL-cholesterol, HDL-cholesterol, and TG, were assessed using an enzymatic colourimetric method on Roche/Hitachi Cobas C systems (C111). The reference ranges provided by the manufacturer in the kit insert were used as benchmarks. The method described by Lowry et al. [23] was employed to determine the total protein content, and the absorbances were measured using a UV–Visible spectrophotometer (Multiscan Go, Thermo-Fisher Scientific, MA, USA).
2.4. ELISA
FFA and diacylglycerol (DAG) were analysed using rat ELISA kits per the manufacturer's protocol. The insulin content in serum and organs was also analysed using Rat Ins1/Insulin ELISA kit according to the manufacturer's protocol.
2.5. Glutathione analysis
The reduced glutathione (GSH) content was analysed in serum and organs using the protocol described earlier [24]. Briefly, 100 μl of serum or 100 μl of a 10% organ homogenate in phosphate-buffered saline was mixed with 100 μl of 20% trichloroacetic acid (TCA) (vol/vol) for precipitation. After centrifugation at 5000 rpm for 10 min, 100 μl of the supernatant was collected and mixed with 100 μl of 0.2 M tris buffer (pH 8.9, substituted with 0.2 M EDTA solution in the ratio of 10:1), followed by 5 μl of 0.01 M 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB) reagent. The sample's absorbance at 412 nm was read against a blank using a UV–Visible spectrophotometer within 5 min of adding the DNTB reagent. The GSH content in plasma was expressed as nmol/ml or nmol/mg tissue.
2.6. Malondialdehyde assay
Malondialdehyde (MDA) was assayed using a modified thiobarbituric acid reactive substances (TBARS) procedure [25]. In this method, 400 μl of plasma or 400 μl of a 10% tissue homogenate was combined with 400 μl of 20% TCA for precipitation. The mixture was centrifuged at 10,000×g for 10 min, and the resulting 400 μl supernatant was collected. Subsequently, the supernatant was incubated with 400 μl of 0.4% thiobarbituric acid (TBA) (w/v in 0.2 N HCl) at a temperature of 60 °C for 1 h. The formed TBA-MDA adducts in the sample were quantified using a Waters Breeze HPLC System (Waters, USA). The HPLC analysis employed a mobile phase consisting of methanol:0.05 M KH2PO4 buffer (pH 6.8) in a ratio of 40:60 (v/v), supplemented with 0.2% (v/v) triethanolamine. A reverse-phase column, Xterra MS C18, with a pore size of 5 μm, was utilized for the analysis. The column temperature was set at 35 °C, and a flow rate of 1 ml/min was maintained, with an injection volume of 20 μl. Fluorescence detection was performed at excitation and emission wavelengths of 532 nm and 553 nm, respectively. The MDA content was determined by calculating the peak area, with malondialdehyde-bis-(diethyl acetal) serving as the standard for the experiment.
2.7. Protein carbonyl assay
Protein-bound carbonyls were measured in serum and tissue homogenates using the method outlined by Castegna et al. [26]. In brief, 100 μl of the sample was mixed with either 400 μl of 2.5 M HCl (control) or 400 μl of 0.2% 2,4-dinitrophenylhydrazine in 2.5 M HCl (test). The mixture was vortexed and incubated at room temperature for 1 h. After the incubation period, protein precipitation was carried out by adding 500 μl of 20% TCA. Following centrifugation at 10,000 rpm for 10 min, the supernatant was discarded. The protein pellet in the tube was washed three times using a wash solution (ethanol: ethyl acetate) in a 1:1 ratio (vol/vol), each wash using 500 μl. Subsequently, the protein pellet was dissolved in 400 μl of 6 M guanidine HCl in sodium phosphate buffer (pH 6.5). The absorbance of the control and test samples was measured at 370 nm using a UV–Visible spectrophotometer. The results were expressed as nmol carbonyl groups per mg protein.
2.8. Statistical analyses
The statistical analyses of the experiments were conducted using GraphPad Prism software. All experiments were carried out in triplicates. To assess the normality assumptions of the data, Shapiro-Wilk's test was applied, and it was found that five datasets (GSH in heart and pancreas, insulin in serum and pancreas, and protein carbonyl in the pancreas) violated the normality assumptions. The non-parametric Kruskal-Wallis test was employed for these treatment groups, and subsequent multiple comparisons were performed using Dunn's test. One-way ANOVA with Tukey test comparisons was utilized to evaluate the treatment effect for all other quantitative parameters. A p-value of less than 0.05 was considered statistically significant.
3. Results
This study's primary objective was to determine if FA supplementation before or after the induction of type 2 DM in rats could alleviate disease-related symptoms such as hyperglycemia and altered lipid profile. For this purpose, serum biochemical analysis was carried out in the animals to record the changes at baseline and the end of the study in PRE-STZ, POST-STZ and STZ groups. DAG and FFA were further analysed to determine if FA can modulate lipid metabolism. The secondary objective was documented by measuring MDA, protein-bound carbonyls and GSH in the treatment groups to understand if FA supplementation can impact lipid and protein oxidative damages in type 2 DM and get preliminary data regarding a potential amelioration of the oxidative status by FA.
3.1. Biochemical assay
The results of the biochemical assay are described in Table 2. Glucose level was significantly elevated in the POST-STZ FA supplemented group compared to the STZ control group. At the same time, no significant difference was observed between the STZ and PRE-STZ FA-supplemented groups. The lipid profile of animals also registered surprising results, where the total cholesterol, HDL, and LDL levels recorded no significant difference in the PRE-STZ and POST-STZ FA-supplemented groups compared to the STZ control group. However, TG was elevated significantly in the PRE-STZ FA-supplemented group compared to the STZ control group and POST-STZ FA-supplemented group. Our results indicate that FA supplementation did not significantly affect the glucose or lipid profile in diabetic rats. In the pre-treated group, the toxicity of STZ was not prevented; therefore, the glucose level, total cholesterol, and TG increased significantly compared to the control group. No significant insulin differences were observed in serum, liver and pancreas between the 3 study groups (Fig. 1).
Table 2.
Serum biochemical parameters.
| STZ (n = 6) | PRE-STZ (n = 6) | POST-STZ (n = 6) | |
|---|---|---|---|
| Glucose (mg/dl) | 559.07 ± 13.77 | 623.47 ± 19.45 | 654.70 ± 24.08a |
| Total Cholesterol (mg/dl) | 68.48 ± 4.33 | 85.67 ± 5.40 | 71.42 ± 2.70 |
| Triglycerides (mg/dl) | 115.37 ± 11.92 | 263.78 ± 9.71a | 166.65 ± 25.88a,b |
| LDL (mmol/l) | 0.33 ± 0.02 | 0.28 ± 0.02 | 0.29 ± 0.03 |
| HDL (mg/dl) | 44.88 ± 0.78 | 57.81 ± 2.87 | 45.96 ± 1.75 |
STZ (unsupplemented control group), PRE-STZ (ferulic acid pre-treated group), POST-STZ (ferulic acid supplemented after STZ treatment). Data presented as mean ± SEM. ANOVA with Tukey test was used to assess treatment group differences. Statistical significance set at p ≤ 0.05. a-statistical difference with the control group; b-statistical difference with PRE-STZ group.
Fig. 1.
Insulin Levels in Rat Tissue. Panels A – serum, B – pancreas, C – liver. STZ (unsupplemented control group, n = 6), PRE-STZ (ferulic acid pre-treated group, n = 6), POST-STZ (ferulic acid supplemented after STZ treatment, n = 6). Data presented as mean ± SEM. Serum and pancreas data were analysed by the Kruskal-Wallis test, and multiple comparisons were performed with Dunn's test. ANOVA with multiple comparisons using the Tukey test was performed on liver data. Statistical significance set at p ≤ 0.05. ns-no significant difference.
3.2. FFA assay
Although the difference was insignificant, the FFA level in the PRE-STZ group's serum was higher than in the STZ control group (Fig. 2). The result observed in serum agrees with the elevated triglyceride levels in the PRE-STZ group. A similar upward trend was seen in the FFA levels of the PRE-STZ group and POST-STZ group of pancreas and liver, respectively. However, it was not statistically significant. We observed no changes in the heart FFA content with FA supplementation.
Fig. 2.
Free Fatty Acid Levels in Rat Tissue. Panels A – serum, B – heart, C – liver, D - pancreas. STZ (unsupplemented control group, n = 6), PRE-STZ (ferulic acid pre-treated group, n = 6), POST-STZ (ferulic acid supplemented after STZ treatment, n = 6). Data presented as mean ± SEM. ANOVA with multiple comparisons using the Tukey test was performed for all quantitative parameters to identify the test groups' treatment effect. Statistical significance set at p ≤ 0.05. ns-no significant difference.
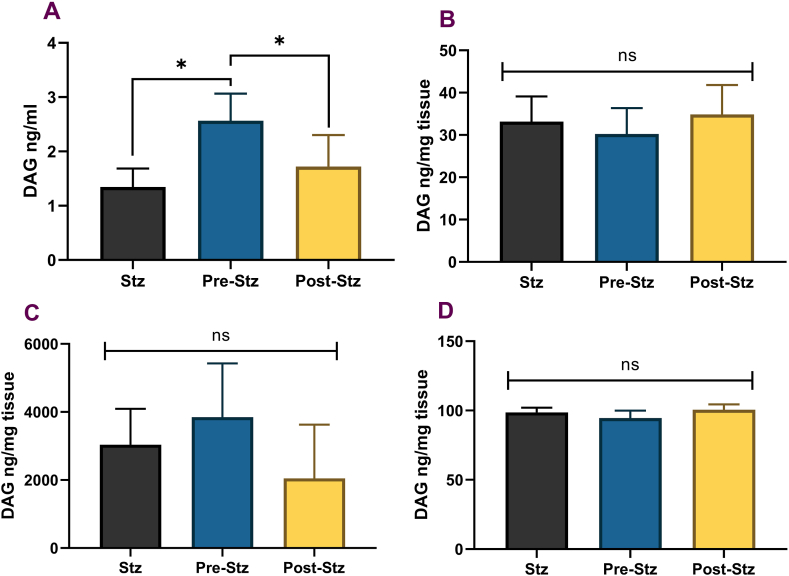
3.3. DAG assay
FA supplementation before or after the induction of DM did not alter DAG levels in rats' heart and liver tissue, and the observed changes in DAG content were not significant in the pancreas (Fig. 3). However, circulating DAG levels were significantly elevated in the PRE-STZ group compared to STZ and POST-STZ groups. The DAG levels in the POST-STZ group were similar to the STZ group.
Fig. 3.
Diacylglycerol Levels in Rat Tissue. Panels A – serum, B – heart, C – pancreas, D – liver. STZ (unsupplemented control group, n = 6), PRE-STZ (ferulic acid pre-treated group, n = 6), POST-STZ (ferulic acid supplemented after STZ treatment, n = 6). Data presented as mean ± SEM. ANOVA with multiple comparisons using the Tukey test was performed for all quantitative parameters to identify the test groups' treatment effect. Statistical significance set at p ≤ 0.05. * significant difference, ns-no significant difference.
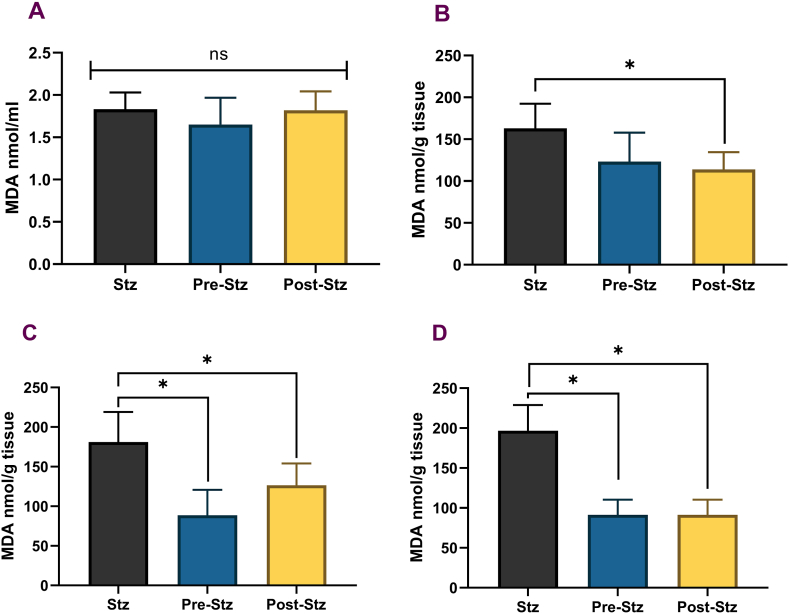
3.4. Lipid and protein oxidative damage and GSH
There was a significant reduction of oxidative damage biomarkers, MDA, in the pancreas and liver (Fig. 4). The MDA levels were significantly reduced in POST-STZ and PRE-STZ groups compared to the STZ control. The same trend was observed in the heart, but the data was only significant for POST-STZ than STZ. There was no significant difference between the pre-and post-treated groups, and FA supplementation did not improve the serum MDA levels. The measured protein-bound carbonyl levels in the serum and tissues were similar to MDA levels. No significant difference was observed between the test groups in the serum, and similarly, the levels did not change in the animals' heart tissues. However, protein oxidation levels were significantly lowered in the liver and pancreas (Fig. 5). The protein carbonyl levels in the liver and heart were significantly reduced in PRE-STZ and POST-STZ groups with FA supplementation. However, there was no significant difference between the pre-and post-treated groups in liver and heart tissue. FA supplementation in PRE-STZ or POST-STZ animals did not significantly differ in the GSH levels in heart or liver tissue than the STZ group (Fig. 6). There was a general increase in GSH content in serum and pancreas, where the level was highest in POST-STZ, intermediate in PRE-STZ and lowest in STZ groups; however, the differences were not significant except for the POST-STZ group in the pancreas. The level of reduced glutathione was significantly elevated in the pancreatic tissue of POST-STZ compared to STZ and PRE-STZ groups.
Fig. 4.
Lipid Peroxidation in Rat Tissue. Panels A – serum, B – heart, C – pancreas, D – liver. STZ (unsupplemented control group, n = 6), PRE-STZ (ferulic acid pre-treated group, n = 6), POST-STZ (ferulic acid supplemented after STZ treatment, n = 6). Data presented as mean ± SEM. ANOVA with multiple comparisons using the Tukey test was performed for all quantitative parameters to identify the test groups' treatment effect. Statistical significance set at p ≤ 0.05. * significant difference, ns-no significant difference.
Fig. 5.
Protein-bound Carbonyls in Rat Tissue. Panels A – serum, B – heart, C – liver, D – pancreas. STZ (unsupplemented control group, n = 6), PRE-STZ (ferulic acid pre-treated group, n = 6), POST-STZ (ferulic acid supplemented after STZ treatment, n = 6). Data presented as mean ± SEM. Pancreas data were analysed by the Kruskal-Wallis test, and multiple comparisons were performed with Dunn's test. ANOVA with multiple comparisons using the Tukey test was performed in serum, liver and heart data. Statistical significance set at p ≤ 0.05. * significant difference, ns-no significant difference.
Fig. 6.
Glutathione Levels in Rat Tissue. Panels A – serum, B – heart, C – pancreas, D – liver. STZ (unsupplemented control group, n = 6), PRE-STZ (ferulic acid pre-treated group, n = 6), POST-STZ (ferulic acid supplemented after STZ treatment, n = 6). Data presented as mean ± SEM. Heart and pancreas data were analysed by the Kruskal-Wallis test, and multiple comparisons were performed with Dunn's test. ANOVA with multiple comparisons using the Tukey test was performed in the liver and serum. Statistical significance set at p ≤ 0.05. * significant difference, ns-no significant difference.
4. Discussion
This study examined the effects of a preventive supplementation of FA in diabetic rat models. One key strength of the work is the novelty of its study design. Study models exploring the differences between preventive and therapeutic supplements have not been hitherto explained. We examined how FA's preventive supplementation differed from therapeutic supplementation in STZ-induced diabetic rats. Our study's results indicated no improvement in the serum biochemical parameters such as blood glucose level and lipid profile with FA supplementation, indicating that FA failed to modulate the symptoms of diabetes positively; hence the hypothesis was rejected. Interestingly, several studies have reported positive improvements in biochemical parameters such as blood glucose and lipid profile in diabetic models with FA supplementation [12,13,17,20]. One critical factor that governs polyphenols' efficacy is their bioavailability [27]. FA may influence serum glucose level and lipid profile by preventing STZ toxicity, thereby improving insulin secretion by the pancreatic beta cells. The FA must reach the target tissue in sufficient concentrations to have biological action. A possible reason for our result could be the low bioavailability of ferulic acid. A study conducted in rats to characterise FA reports evidence of FA in the liver, kidney, spleen and lung 2 h following oral administration [28]. A large portion of the FA remained in the stomach after 2 h, indicating slower absorption. Following absorption of FA in rats, three metabolites have been reported, all glucuronides of FA. It also suggests that the metabolism rate is slow, and the hydroxycinnamate is primarily excreted as its parent compound in the urine. A recent study by Chen et al. described FA's pharmacokinetics in rats after consuming Polygonum Chinese Linn extract [29]. This study reported the time of peak plasma value for FA after ingestion of a dose of polyphenol source equivalent to FA dose of 3.30 g/kg body weight was more than an hour with the peak plasma value of 1112 ± 25 ng/ml, suggesting that the compound is slowly absorbed. Besides, the low volume of distribution of FA from their study also suggests the weak transport of FA into various tissues such as the heart, liver, spleen, lung and kidney. However, another pharmacokinetic study of ferulic acid reports that FA was rapidly absorbed following oral administration reaching peak plasma concentration within 1.8 min, and the plasma concentrations neared zero in 3 h [30]. The authors demonstrated the low bioavailability of ferulic acid due to quick absorption and clearance and highlighted how it improved with the co-administration of other agents such as Honghua (Chinese herb) and clopidogrel. Although there are discrepancies in the Cmax, AUC and Tmax values of ferulic acid from various pharmacokinetic studies, all of them demonstrate the low bioavailability of the compound. Li et al. suggest that these discrepancies could be due to the different solvents used to administer FA [31]. Moreover, an alternate route of administration has been suggested, such as transdermal administration, to enable the slow and controlled release of ferulic acid into circulation for therapeutic purposes [32]. These observations reinforce the question of bioavailability in modulating positive outcomes in disease states.
Impairment of glucose levels has wide-ranging consequences in the human body. Increased glucose levels in the serum during diabetes increase tissue fatty acid synthesis [28]. Any dysregulation in glucose metabolism affects the metabolism of fats, and the most notable feature of diabetes is the increase in FFA in the serum, which increases the risk of cardiovascular disease. FFA level remained unchanged in the heart tissue of both treatment groups compared to the STZ group, indicating no notable difference in tissue with FFA supplementation. Metabolomic study in rats tissue after 2 h following oral intake revealed that although FA was widely distributed in various tissues such as the liver, kidney, spleen, and even lung, there was no trace of FA in heart tissue [28]. The low bioavailability of FA might explain the unchanged levels of FFA in the heart. DAG levels are elevated in diabetes due to an increase in glycolytic intermediate glycerol-3-phosphate, which increases the synthesis of DAG. In diabetes, the total DAG levels are elevated in tissues of the retina, aorta, heart, liver, and even skeletal muscles, where it activates protein kinase C. Consequently, modulation of various cellular and vascular processes occurs, such as endothelial dysfunction, increased vascular permeability, basement membrane thickening and extracellular matrix expansion [33]. In the current study, the FA supplementation's inability to reduce glucose levels directly affected DAG levels in the animals. Moreover, the elevated levels of DAG observed in serum are consistent with the elevated levels of TG and FFA in serum in the PRE-STZ group, although the elevations of TG and FFA did not reach significance.
The pronounced effect of FA supplementation was observed on oxidative damage in the tissue. FA supplementation decreased lipid peroxidation by-product MDA in the liver and pancreas in both PRE-STZ and POST-STZ groups compared to the control STZ group. In the case of heart tissue, only POST-STZ was significantly lowered. A dose-dependent decrease in measured MDA levels in liver tissue was reported in rats with FA supplementation [18]. Our study has a consensus between the measured levels of the two oxidative damage biomarkers, MDA and protein-bound carbonyls. The protein-bound carbonyls, generated from protein oxidation in the animals, were also lowered in the liver and pancreatic tissue with FA supplementation. The carbonyl groups were significantly reduced in the liver for both PRE-STZ and POST-STZ groups and only the PRE-STZ group in the pancreas. There are several earlier reports on FA's ability to prevent the oxidation of proteins in vitro and in vivo [[34], [35], [36]]. Protein-bound carbonyls are critical intermediates forming advanced glycation end products like carboxymethyl lysine and pentosidine in DM [37]. Hence, the disease is characterised by increased protein-bound carbonyl levels in circulation [38]. The decrease of these biomarkers in tissue indicates the FA's ability to reverse oxidative damage associated with DM. The antioxidant effect of FA depends primarily on the hydroxyl and methoxy groups attached to the phenyl ring in its structure. Several previous studies concluded about the ability of FA to improve antioxidant status [17,20,21,39]. In the present work, while FA positively impacted lipid and protein oxidative damages, no increase in the glutathione level was observed except in the pancreatic tissue in the POST-STZ group. The data agrees with the conclusions of Balasubhashini et al., 2004, where it was suggested that the improvements observed with FA supplementation in DM animal models could be attributed to its ability to neutralise free radicals generated by STZ thereby reducing its toxicity in the tissue [39]. The GSH result in the POST-STZ group indicates a similar mechanism whereby the FA has improved the tissue's resistance to free radical damage.
Our study's results could not clearly define the differences between FA's preventive and therapeutic effects. The difference in the duration of FA supplementation between the PRE-STZ and POST-STZ groups was two weeks. Chowdhury et al. demonstrated improvements in glucose regulation as early as 2–3 weeks with doses of 50–70 mg/kg body weight FA in diabetic rats [16]. However, in our study, we did not observe similar results. One of our study's main limitations is the lack of data from the blood sample of the PRE-STZ group before STZ treatment. This limits our study's potential to explain any observable improvements in the antioxidant status that occurred in the animals with FA supplementation before DM induction; hence future research designs can include sampling throughout the supplementation period to account for these changes. Additionally, based on our results, an increase in the difference in FA supplementation duration between PRE-STZ and POST-STZ groups is advised in future studies. The observed improvement in antioxidant status with FA supplementation indicates that FA can bring about tissue-level improvements even without systemic changes related to type-2 diabetes. However, further research is warranted to elucidate and confirm whether the observed improvement in the antioxidant status is due to FA's free radical quenching ability and/or if FA was activating the Antioxidant Response Element.
5. Conclusions
Our study results are coherent globally after factoring in ferulic acid bioavailability. While the amount of FA that may reach the animals' tissues effectively improved lipid and protein oxidative damages, it did not bring about notable changes in disease biomarkers of type 2 DM such as blood glucose, lipid profile, FFA or DAG. It can be inferred that while the quantities of FA in tissue may have been sufficient to counter some of the damages related to oxidative stress, it did not modulate any molecular targets in the tissue for improved glucose regulation. Hence, any influence of FA supplementation in the pre-treated groups could not be clearly defined compared to the post-treated group in the study.
Author contribution statement
Serene Hilary: Performed the experiments; Analysed and interpreted the data; Wrote the paper. Ozaz Mohamed, Jaleel Kizhakkayil: Performed the experiments; Wrote the paper. Carine Platat, Frank C Howarth: Conceived and designed the experiments; Analysed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Muhammad Qureshi, Fatima Al-Meqbaali: Performed the experiments.
Funding statement
This study was financially supported by the United Arab Emirates University [grant number 31M371].
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kaur J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014;2014 doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Behl T., Kotwani A. Proposed mechanisms of Terminalia catappa in hyperglycaemia and associated diabetic complications. J. Pharm. Pharmacol. 2017;69(2):123–134. doi: 10.1111/jphp.12676. [DOI] [PubMed] [Google Scholar]
- 3.Hui Y., Xun J., Christopher Wai Kei L., Sheng-Kai Y. Oxidative stress and diabetes mellitus. Clin. Chem. Lab. Med. 2011;49(11):1773–1782. doi: 10.1515/CCLM.2011.250. [DOI] [PubMed] [Google Scholar]
- 4.Kalofoutis C., Piperi C., Kalofoutis A., Harris F., Phoenix D., Singh J. Type II diabetes mellitus and cardiovascular risk factors: current therapeutic approaches. Exp. Clin. Cardiol. 2007;12 [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao J.B., Hogger P. Dietary polyphenols and type 2 diabetes: current insights and future perspectives. Curr. Med. Chem. 2015;22(1):23–38. doi: 10.2174/0929867321666140706130807. [DOI] [PubMed] [Google Scholar]
- 6.Mirmiran P., Noori N., Zavareh M.B., Azizi F. Fruit and vegetable consumption and risk factors for cardiovascular disease. Metabolism. 2009;58(4):460–468. doi: 10.1016/j.metabol.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Huang W.-Y., Cai Y.-Z., Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr. Cancer. 2009;62(1):1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 8.Rossi L., Mazzitelli S., Arciello M., Capo C.R., Rotilio G. Benefits from dietary polyphenols for brain aging and Alzheimer's disease. Neurochem. Res. 2008;33(12):2390. doi: 10.1007/s11064-008-9696-7. [DOI] [PubMed] [Google Scholar]
- 9.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2 doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hokayem M., Blond E., Vidal H., Lambert K., Meugnier E., Feillet-Coudray C., et al. Grape polyphenols prevent fructose-induced oxidative stress and insulin resistance in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2013;36(6):1454–1461. doi: 10.2337/dc12-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srinivasan M., Sudheer A.R., Menon V.P. Ferulic Acid: therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007;40(2):92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung E.H., Kim S.R., Hwang I.K., Ha T.Y. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J. Agric. Food Chem. 2007;55 doi: 10.1021/jf0714463. [DOI] [PubMed] [Google Scholar]
- 13.Ohnishi M., Matuo T., Tsuno T., Hosoda A., Nomura E., Taniguchi H., et al. Antioxidant activity and hypoglycemic effect of ferulic acid in STZ-induced diabetic mice and KK-Ay mice. Biofactors. 2004;21(1–4):315–319. doi: 10.1002/biof.552210161. [DOI] [PubMed] [Google Scholar]
- 14.Yogeeta S.K., Gnanapragasam A., Kumar S.S., Subhashini R., Sathivel A., Devaki T. Synergistic interactions of ferulic acid with ascorbic acid: its cardioprotective role during isoproterenol induced myocardial infarction in rats. Mol. Cell. Biochem. 2006;283(1):139–146. doi: 10.1007/s11010-006-2494-0. [DOI] [PubMed] [Google Scholar]
- 15.Luo C., Zhang Y., Guo H., Han X., Ren J., Liu J. Ferulic acid attenuates hypoxia/reoxygenation injury by suppressing mitophagy through the PINK1/parkin signaling pathway in H9c2 cells. Front. Pharmacol. 2020;11:103. doi: 10.3389/fphar.2020.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowdhury S., Ghosh S., Das A.K., Sil P.C. Ferulic acid protects hyperglycemia-induced kidney damage by regulating oxidative insult, inflammation and autophagy. Front. Pharmacol. 2019;10:27. doi: 10.3389/fphar.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song Y., Wu T., Yang Q., Chen X., Wang M., Wang Y., et al. Ferulic acid alleviates the symptoms of diabetes in obese rats. J. Funct. Foods. 2014;9:141–147. [Google Scholar]
- 18.Jain P.G., Surana S.J. Isolation, characterization and hypolipidemic activity of ferulic acid in high-fat-diet-induced hyperlipidemia in laboratory rats. EXCLI J. 2016;15:599. doi: 10.17179/excli2016-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prabhakar P.K., Prasad R., Ali S., Doble M. Synergistic interaction of ferulic acid with commercial hypoglycemic drugs in streptozotocin induced diabetic rats. Phytomedicine. 2013;20(6):488–494. doi: 10.1016/j.phymed.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Roy S., Metya S.K., Sannigrahi S., Rahaman N., Ahmed F. Treatment with ferulic acid to rats with streptozotocin-induced diabetes: effects on oxidative stress, pro-inflammatory cytokines, and apoptosis in the pancreatic β cell. Endocrine. 2013;44(2):369–379. doi: 10.1007/s12020-012-9868-8. [DOI] [PubMed] [Google Scholar]
- 21.Ramar M., Manikandan B., Raman T., Priyadarsini A., Palanisamy S., Velayudam M., et al. Protective effect of ferulic acid and resveratrol against alloxan-induced diabetes in mice. Eur. J. Pharmacol. 2012;690(1–3):226–235. doi: 10.1016/j.ejphar.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Narasimhan A., Chinnaiyan M., Karundevi B. Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metabol. 2015;40(8):769–781. doi: 10.1139/apnm-2015-0002. [DOI] [PubMed] [Google Scholar]
- 23.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 24.Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 25.Hilary S., Habib H., Souka U., Ibrahim W., Platat C. Bioactivity of arid region honey: an in vitro study. BMC Compl. Alternative Med. 2017;17(1):177. doi: 10.1186/s12906-017-1664-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castegna A., Drake J., Pocernich C., Butterfield D.A. Springer; 2003. Protein Carbonyl Levels—An Assessment of Protein Oxidation. Methods in Biological Oxidative Stress; pp. 161–168. [Google Scholar]
- 27.Di Lorenzo C., Colombo F., Biella S., Stockley C., Restani P. Polyphenols and human health: the role of bioavailability. Nutrients. 2021;13(1):273. doi: 10.3390/nu13010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J.-L., Zhang G.-D., Zhou T.-H. Metabolism of ferulic acid in rats. J. Asian Nat. Prod. Res. 2005;7(1):49–58. doi: 10.1080/10286020310001617129. [DOI] [PubMed] [Google Scholar]
- 29.Chen X., Wei L., Pu X., Wang Y., Xu Y. Pharmacokinetics and tissue distribution study of 15 ingredients of Polygonum chinense Linn extract in rats by UHPLC–MS/MS. Biomed. Chromatogr. 2020:e4975. doi: 10.1002/bmc.4975. [DOI] [PubMed] [Google Scholar]
- 30.Li Y., Liu C., Zhang Y., Mi S., Wang N. Pharmacokinetics of ferulic acid and potential interactions with Honghua and clopidogrel in rats. J. Ethnopharmacol. 2011;137(1):562–567. doi: 10.1016/j.jep.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Li D., Rui Y-x, Guo S-d, Luan F., Liu R., Zeng N. Ferulic acid: a review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021;284 doi: 10.1016/j.lfs.2021.119921. [DOI] [PubMed] [Google Scholar]
- 32.Yan N., Tang Z., Xu Y., Li X., Wang Q. Pharmacokinetic study of ferulic acid following transdermal or intragastric administration in rats. AAPS PharmSciTech. 2020;21(5):169. doi: 10.1208/s12249-020-01709-w. [DOI] [PubMed] [Google Scholar]
- 33.Geraldes P., King G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010;106(8):1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferulic acid supplements abrogate oxidative impairments in liver and testis in the streptozotocin-diabetic rat. Zool. Sci. 2008;25(8):854–860. doi: 10.2108/zsj.25.854. [DOI] [PubMed] [Google Scholar]
- 35.Sompong W., Cheng H., Adisakwattana S. Ferulic acid prevents methylglyoxal-induced protein glycation, DNA damage, and apoptosis in pancreatic β-cells. J. Physiol. Biochem. 2017;73(1):121–131. doi: 10.1007/s13105-016-0531-3. [DOI] [PubMed] [Google Scholar]
- 36.Sompong W., Meeprom A., Cheng H., Adisakwattana S. A comparative study of ferulic acid on different monosaccharide-mediated protein glycation and oxidative damage in bovine serum albumin. Molecules. 2013;18(11) doi: 10.3390/molecules181113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liggins J., Furth A.J. Role of protein-bound carbonyl groups in the formation of advanced glycation endproducts. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 1997;1361(2):123–130. doi: 10.1016/s0925-4439(97)00023-9. [DOI] [PubMed] [Google Scholar]
- 38.Colombo G., Reggiani F., Cucchiari D., Astori E., Garavaglia M.L., Portinaro N.M., et al. Plasma protein carbonylation in haemodialysed patients: focus on diabetes and gender. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/4149681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balasubashini M.S., Rukkumani R., Viswanathan P., Menon V.P. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Derivat. 2004;18(4):310–314. doi: 10.1002/ptr.1440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.