Graphical abstract
Mechanism of ALA/LNA ratios on muscle glucose metabolism and fatty acid metabolism.
Keywords: Ctenopharyngodon idella, ALA/LNA ratio, Fatty acid metabolism, Glucose metabolism, Flesh quality
Highlights
-
•
ALA/LNA ratios enhanced growth performance and meat quality of fish.
-
•
ALA/LNA ratios improved lipid metabolism and transport of fish.
-
•
ALA/LNA ratios improved beneficial fatty acid composition of fish.
-
•
ALA/LNA ratios increased UFA content by influencing biosynthesis and catabolism.
-
•
ALA/LNA ratios enhanced muscle glucose uptake and utilization of fish.
Abstract
The n6/n3 ratios improved meat quality of terrestrial animals, but alpha-linolenic acid/linoleic acid (ALA/LNA) ratios were rarely studied in aquatic animals. In this study, sub-adult grass carp (Ctenopharyngodon idella) were fed diets fed diets containing six varying ALA/LNA ratios (0.03, 0.47, 0.92, 1.33, 1.69, and 2.15) for 9 weeks and the total value of n3 + n6 (1.98) was kept constant for all six treatments. The results indicated optimal ALA/LNA ratio improved growth performance, changed fatty acid composition in grass carp muscle, and promoted glucose metabolism. Additionally, optimal ALA/LNA ratio improved chemical attributes by increasing crude protein and lipid contents, and technological attributes by increasing pH24h value and shear force in grass carp muscle. The signaling pathways related to fatty acid metabolism and glucose metabolism (LXRα/SREBP-1, PPARα, PPARγ, AMPK) might be responsible for these changes. Dietary optimal ALA/LNA ratio based on PWG, UFA and glucose contents was 1.03, 0.88 and 0.92, respectively.
1. Introduction
With the development of society and the improvement of the economic level, people are becoming more and more concerned about flesh quality. Flesh quality properties include chemical properties (protein, moisture, and lipid), sensory properties (color, tenderness, and flavor), and technological attributes (pH and shear force) (Xiong, Sun, Zeng, & Xie, 2014). Flesh quality is easily affected by nutrients such as protein, lipid (especially fatty acids), and amino acids (Munekata, Pateiro, Lopez-Pedrouso, Gagaoua, & Lorenzo, 2021). Appropriate n6/n3 ratio improved meat color and pH24h value in Heigai pigs (Nong et al., 2020). Alpha-linolenic acid (ALA, 18:3n-3) and linoleic acid (LNA, 18:2n-6) are essential fatty acids (EFAs) for all freshwater fish as vital members of n-3 and n-6 fatty acids, respectively (Glencross, 2009). Studies found optimal ALA/LNA ratio enhanced the growth performance of juvenile grass carp (Ctenopharyngodon idella) in our laboratory (Zeng et al., 2016) and changed fatty acid composition of silver barb (Puntius gonionotus) liver and muscle (Nayak, Giri, Pradhan, Samanta, & Saha, 2020). Meat quality indicators are affected by biochemical reactions such as fatty acid and glucose metabolism. However, the influences of dietary ALA/LNA ratios on flesh quality, fatty acid and glucose metabolism in animal muscle are limited, and remain to be studied.
Meat quality is affected by fatty acid composition. Fatty acid anabolism and catabolism can determine the fatty acid composition of fish tissues. Fatty acids are classified as saturated fatty acid (SFA), monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA). The synthesis of SFA, MUFA, and PUFA requires fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC) and FAS, ACC, stearoyl-CoA desaturase 1 (SCD1), as well as Δ6 desaturase (Δ6 Fad) and elongase (such as Elovl5), respectively. These enzymes are modulated by liver X receptor α (LXRα)/sterol regulatory element binding protein-1 (SREBP-1) (Li, Zhang, Qiu, Deng, & Wang, 2022). Peroxisome proliferator-activated receptor α (PPARα) also regulates Δ6Fad and Elovl (Xie et al., 2021).To our knowledge, there were two studies about the influence of ALA/LNA ratios on fatty acid synthesis in fish muscle, which focused on the effect of ALA/LNA ratios on FAS mRNA level in large yellow croaker (Larimichthys crocea) muscle (Zuo, Mai, Xu, Turchini, & Ai, 2015) and Δ6 fad, Elovl5 mRNA levels in silver barb muscle (Nayak, Giri, Pradhan, Samanta, & Saha, 2020). However, no study has deeply investigated the influences of dietary ALA/LNA ratios on fatty acid synthesis and related signaling pathways in fish. Study observed that optimal ALA/LNA ratio increased target of rapamycin (TOR) mRNA level and leptin concentration in juvenile grass carp intestine (Zeng et al., 2016). mTOR increased SREBP-1 mRNA level in rat muscle (Joshi et al., 2013). Leptin significantly boosted PPARs DNA-binding activity of C2C12 myoblasts in mice (Bendinelli, Piccoletti, & Maroni, 2005). These studies showed that dietary ALA/LNA ratios might affect fatty acid synthesis in fish, which needs further investigation.
Except for fatty acid synthesis, fatty acid catabolism also affects fatty acid contents. Fatty acid catabolism requires acyl coenzyme A oxidase (ACO) and carnitine palmitoyl transferase 1 (CPT1), which can be modulated by peroxisome proliferator-activated receptor alpha (PPARα) (Li, Zhang, Qiu, Deng, & Wang, 2022). So far, there has been one research that focused on the effect of ALA/LNA ratios on PPARα, ACO, and CPT1 mRNA levels in large yellow croaker (marine fish) muscle (Zuo, Mai, Xu, Turchini, & Ai, 2015). But no study has explored the influences of dietary ALA/LNA ratios on fatty acid catabolism in freshwater fish. There are two differences between marine fish and freshwater fish in terms of fatty acid catabolism. Firstly, the EFAs (EPA and DHA) of marine fish are different from those of freshwater fish (ALA and LNA) (Glencross, 2009). Secondly, the CPT1 subtypes of large yellow croaker (CPT1a) are different from those of grass carp (CPT1α1a, 1α1b, 1α2a, 1α2b, and 1β) (Wang et al., 2019, Shi et al., 2017). These studies showed that fatty acid catabolism in marine and freshwater fish might be different. Therefore, it is necessary to study the influence of dietary ALA/LNA ratios on fatty acid catabolism in freshwater fish.
Fatty acid catabolism affects glucose metabolism. Glucose can enter muscle tissue via glucose transporters (GLUT1 and GLUT4) and be converted into muscle glycogen, which can enter the pentose phosphate pathway in the presence of glucose-6-phosphate dehydrogenase (G6PD) (Injarabian et al., 2020, Wang et al., 2021). AMP-activated protein kinase (AMPK) promotes glucose uptake and utilization in fish skeletal muscle (Magnoni, Vraskou, Palstra, & Planas, 2012). So far, there has been one study that studied the effect of ALA/LNA ratios on the mRNA level of G6PD in large yellow croaker (carnivorous fish) muscle (Zuo, Mai, Xu, Turchini, & Ai, 2015). Nevertheless, no research has studied the influences of dietary ALA/LNA ratios on glucose metabolism in herbivorous fish. Research discovered that herbivorous fish could use more carbohydrates than carnivorous fish (Zhou, Wang, Xie, Deng, & Zhou, 2016). LNA stimulated insulin release in rat pancreas (Lai, Teng, & Yang, 2013). Insulin increased glycogen synthesis in mice skeletal muscle (Bouskila, Hirshman, Jensen, Goodyear, & Sakamoto, 2008). ALA/LNA ratios increased plasma estrogen (E2) levels in juvenile common carp (Cyprinus carpio) (Ma et al., 2020). E2 elevated protein levels of GLUT1, GLUT4 and p-AMPK/AMPK in cattle polymorphonuclear neutrophils (Wang et al., 2021). Nevertheless, whether dietary ALA/LNA ratios affect glucose metabolism in herbivorous fish is unknown and needs further study.
Grass carp is popular with consumers because of its palatability, nutritional value, and economic value (Zeng et al., 2016). Sub-adult grass carp are at market size. Currently, the optimal ALA/LNA ratio for juvenile stage has been studied (Zeng et al., 2016). However, the optimal ALA/LNA ratio varies with the growth stages of aquatic animals. In Atlantic salmon (Salmo salar), the n6/n3 ratio of 0.33 was beneficial for juvenile growth (1.28 ± 0.1 g) (Berge, Witten, Baeverfjord, Vegusdal, Wadsworth, & Ruyter, 2009), but different n6/n3 ratios had no significant effect on youth stage growth (203 ± 24 g) (Katan, Caballero-Solares, Taylor, Rise, & Parrish, 2019). Therefore, it is important to study the optimal ALA/LNA ratio in sub-adult grass carp.
The purpose of this study was to systematically explore the influence of dietary ALA/LNA ratios on muscle fatty acid metabolism in fish, and firstly investigate the influences of dietary ALA/LNA ratios on muscle glucose metabolism and flesh quality in fish, which could provide theoretical support for dietary ALA/LNA ratios on improving flesh quality. Additionally, the optimal ALA/LNA ratio for sub-adult grass carp was determined to provide guidance for the formulation of commercial feed and the yield of high-quality grass carp.
2. Materials and methods
2.1. Animals
The procedures used in this study were approved by the University of Sichuan Agricultural Animal Care Advisory Committee.
2.2. Experimental diets and feeding
Table S1 shows the formulation and nutrient composition of experimental diets. Six isonitrogenous (26%) and isolipidic (4.27%) experimental diets were formulated. Protein sources included casein, soybean isolate protein, and fish meal. Lipid sources included linseed oil, safflower oil (Sanmark Co., Ltd., Liaoning, China) and coconut oil (Qingdao Haizhiyuan Life Technology Co., Ltd., Shandong, China), and they were used to formulate diets with different ALA/LNA ratios (0.00, 0.50, 1.00, 1.50, 2.00 and 2.50), while keeping the total content of C18 PUFA (ALA + LNA) constant (Zeng et al., 2016). 2,6-di-tert-butyl-4-methylphenol (BHT) was added to diets as an antioxidant (Lima, Takahashi, Tabata, Hattori, Ribeiro, & Moreira, 2019). In the six experimental diets, ALA/LNA final ratios were determined to be 0.03 (control group), 0.47, 0.92, 1.33, 1.69, and 2.15. All the ingredients were mixed, crushed, and kept at −20 °C for the experiment.
Grass carp were purchased from a native fishery (Sichuan, China) and adapted to experimental conditions for 2 weeks. A total of 450 fish (mean weight 680.53 ± 1.16 g) were distributed into 18 cages randomly. There were 25 fish per cage, with 3 cages for each group. Each cage was provided with a 1 m-diameter disc made of 1 mm gauze and placed at the bottom to collect leftover feed. Fish were fed four times every day at 08:00, 11:00, 15:00, and 19:00 for 9 weeks, and leftover feed was collected to calculate feed intake (FI) (Wang et al., 2015). During the trial, the water temperature was 27.1 ± 2.9 °C, the dissolved oxygen was ≥6.0 mg/L, and the pH was approximately 7.0.
2.3. Sampling and analysis
At the termination of the experiment, all fish were fasted for 24 h, counted, and weighed. In each treatment, nine fish were sampled. In order to reduce stress, grass carp were anesthetized with benzocaine (50 mg/L) (Wang et al., 2015). Blood was collected from fish tail vessel, centrifuged, and collected supernatant. The serum was kept at −80 °C for indicators measurement. The fish were killed by a blow to the head, and the hepatopancreas and left and right-side muscles of fish were obtained. The left and right-side muscles were divided into four and two parts, respectively. Prior to biochemical indexes and molecular analysis, two parts of left-side muscle were frozen in liquid nitrogen and placed at −80 °C, and the other two parts were immediately used to measure muscle pH value, shear force, and cooking loss. One part of right-side muscle was used to measure nutrients, and the other one part was immersed in 4% paraformaldehyde for tissue section observation.
Water content was measured by oven-drying method at 105 °C. Crude protein content was measured by Kjeldahl method (N × 6.25). Crude lipid content was tested by ether extraction method. The muscle cooking loss was measured according to the method of Brinker & Reiter (2011). The dorsal muscle in the same position of the dissected fish was accurately weighed and recorded as W1, sealed in PE-bags, placed in a water bath at 70 °C for 20 min and then removed. After it cooled down, the surface was gently wiped to absorb the water and weighed and recorded as W2, and the cooking loss was calculated by the formula of cooking loss = (W1-W2)/W1*100%. Fatty acid composition was analyzed by gas chromatography. Briefly, the crude lipid of freeze-dried muscle powder (200 mg) was extracted with chloroform–methanol mixture, then saponified by adding KOH-methanol solution, followed by adding boron trifluoride-methanol solution to obtain fatty acid methyl esters, extracted by hexane, and finally measured by GC-2010 Plus (Shimadzu, Co., Ltd., Kyoto, Japan) (Huang et al., 2018). A 37-fatty acid methyl ester mix (Sigma-Aldrich, St. Louis, MO, USA) was used as the external standards, and the results were expressed as a percentage of total fatty acids. The pH and shear force of muscle samples were measured according to the method described by Brinker and Reiter (2011).
2.4. Biochemical analysis
Commercial kits were used to measure serum glutamic pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT) activities, total cholesterol (TC), total triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
The hepatopancreas and muscle samples were centrifuged (6000 g, 10 min) after being homogenized in 10 volumes (w/v) of ice-cold physiological saline, and then collected supernatants. According to manufacturer’s instructions, hepatopancreas and muscle FAS, hepatopancreas adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL), muscle fatty acid desaturase (Fad), fatty acid elongase (Elovl), and G6PD were determined (Mlbio, Shanghai, China). Muscle dehydrogenase (LDH), pyruvate kinase (PK), phosphofructokinase (PFK), and hexokinase (HK) activities and lactate content were assayed with kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
2.5. Histological observation and immunohistochemistry
To analyze the contents of glycogen and lipid droplet, hematoxylin-eosin (H & E), oil red O, and periodic acid-schiff (PAS) staining were performed by Servicebio Company (Wuhan, China). H & E and oil red O were used to stain hepatopancreas tissues, and oil red O and PAS were used to stain right-side muscle tissues. Images were observed and captured using the ChemiDocTM Imaging System. Image J software was used to identify and quantify glycogen and lipid droplets. Glycogen and lipid droplets were stained purple and red, respectively.
According to SABC kit (Boster Bioengineering Company. Wuhan, Hubei, China), muscle sections were dewaxed and endogenous enzymes were removed with 3% hydrogen peroxide. Then heat antigen repair was done in a microwave oven. The GLUT4 primary antibody was diluted in PBS at 1:100 and incubated overnight at 4 ℃. Incubation with secondary antibody and then with SABC. Staining was visualized with DAB, and hematoxylin was used for counterstaining before sealing. The ChemiDocTM Imaging System was used to observe stained sections at 400× magnification. Image Pro Plus 6.0 was used to quantify cumulative optical density (IOD). GLUT4 was stained brown-yellow.
2.6. Real-time PCR analysis
The previously procedure of real-time PCR analysis was still used (Wang, et al., 2015). The RNAiso Plus Kit was used to extract total RNA from muscle tissues (Takara, China), and the integrity and purity of RNA were determined by 1% agarose gel electrophoresis and spectrophotometry. Prime Script™ RT reagent Kit (Takara, China) was used to synthesize cDNA. Based on our preliminary experimental results, cDNA loading was normalized by using reference gene β-actin (data not shown). Calculation of expression results using 2−ΔΔCt method. Table S2 is the primer sequences.
2.7. Western blotting
The protein samples obtained from muscle tissues lysis were isolated using SDS-PAGE, moved onto PVDF membranes, and incubated with primary antibodies overnight at 4 °C. Finally, the membranes were incubated with secondary antibody for 2 h. ECL luminous agent (China Bishi Biotechnology Co., LTD) and Image Lab software (version 3.0) were used to visualize and quantify bands. Primary antibodies information are listed in Table S3.
2.8. Statistical analysis
SPSS 21.0 software was used for statistical analysis. Mean ± standard deviation (SD) was used to indicate results, and Duncan’s multiple-range test and one-way analysis of variance (ANOVA) were used to examine means differences. P < 0.05 indicated significant data. Optimal ALA/LNA ratio for sub-adult grass carp was calculated using quadratic regression. Growth performance indictors [percent weight gain (PWG), feed efficiency (FE), condition factor (CF), flesh rate, and slaughter rate] were computed as follows:
PWG (%) = [Final body weight (FBW, g/fish) - Initial body weight (IBW, g/fish)]/IBW (g/fish) × 100;
FE (%) = [FBW (g/fish) - IBW (g/fish)]/FI (g/fish) × 100;
CF (g/cm3) = [FBW (g/fish)/body length (cm)3] × 100;
Flesh rate (%) = [FBW (g/fish) - head weight (g/fish) - tail weight (g/fish) - viscera weight (g/fish) - bone weight (g/fish)]/FBW (g/fish) × 100;
Slaughter rate (%) = [FBW (g/fish) - head weight (g/fish) - tail weight (g/fish) - viscera weight (g/fish)]/FBW (g/fish) × 100.
3. Results and discussion
3.1. Growth performance and hepatopancreas health
As displayed in Table 1, when the ALA/LNA ratio was 0.92, grass carp had higher PWG, FI and FE than control group (P < 0.05). This result indicated that optimal ALA/LNA ratio improved growth performance of grass carp, and the result was consistent with the results of juvenile grass carp (Zeng et al., 2016). The growth status of fish can be reflected by CF. As shown in Table 1, the ratio of 2.15 significantly reduced CF of grass carp compared with the ratio of 0.92 (P < 0.05), probably because the ratio of 2.15 was too high and inhibited the growth of grass carp. In addition, both flesh rate and slaughter rate are important bases for evaluating growth performance and economic value of fish. Table 1 showed that flesh rate and slaughter rate reached the maximum value at the ALA/LNA ratio of 0.92 (P < 0.05). This result supports that the ALA/LNA ratio of 0.92 can improve growth performance of grass carp.
Table 1.
Effects of dietary alpha-linolenic acid/linoleic acid (ALA/LNA) ratios on growth performance, serum biochemical indexes and hepatopancreas enzyme activities of sub-adult grass carp (Ctenopharyngodon idella).
| ALA/LNA ratios | 0.03 | 0.47 | 0.92 | 1.33 | 1.69 | 2.15 |
|---|---|---|---|---|---|---|
| Growth performance | ||||||
| IBW 1 | 680.00 ± 1.60 | 680.27 ± 1.85 | 681.33 ± 1.67 | 680.27 ± 1.22 | 680.27 ± 1.22 | 681.07 ± 0.92 |
| FBW 1 | 1298.13 ± 21.91a | 1497.87 ± 22.49c | 1608.80 ± 19.64d | 1482.13 ± 12.56c | 1385.87 ± 7.26b | 1319.47 ± 3.23a |
| PWG 1 | 90.90 ± 3.09a | 120.19 ± 3.01c | 136.13 ± 3.24d | 117.88 ± 2.19c | 103.72 ± 0.83b | 93.74 ± 0.70a |
| FI 1 | 1122.77 ± 1.75a | 1354.46 ± 0.77e | 1501.28 ± 0.81f | 1329.62 ± 0.86d | 1265.65 ± 1.52c | 1138.67 ± 1.62b |
| FE 1 | 55.06 ± 1.98a | 60.36 ± 1.61b | 61.78 ± 1.36b | 60.31 ± 0.99b | 55.75 ± 0.56a | 56.07 ± 0.33a |
| CF 2 | 1.84 ± 0.07ab | 1.84 ± 0.04ab | 1.89 ± 0.09b | 1.85 ± 0.07ab | 1.82 ± 0.06ab | 1.79 ± 0.10a |
| Flesh rate 2 | 62.45 ± 0.91a | 63.04 ± 0.89ab | 64.18 ± 0.79b | 63.49 ± 1.18ab | 63.12 ± 1.21ab | 63.15 ± 0.44ab |
| Slaughter rate 2 | 65.28 ± 1.11a | 65.71 ± 0.88ab | 66.52 ± 0.85b | 65.91 ± 1.11ab | 65.71 ± 1.01ab | 65.91 ± 0.43ab |
| Serum biochemical indices | ||||||
| TG 2 | 2.36 ± 0.25a | 2.76 ± 0.31b | 3.55 ± 0.26c | 2.70 ± 0.14b | 2.70 ± 0.12b | 2.78 ± 0.15b |
| TC 2 | 8.39 ± 0.57a | 8.91 ± 0.58ab | 9.48 ± 0.54b | 9.05 ± 0.98ab | 8.76 ± 0.57ab | 9.24 ± 0.69ab |
| HDL-C 2 | 2.43 ± 0.15a | 3.33 ± 0.14c | 3.53 ± 0.29c | 2.88 ± 0.20b | 2.87 ± 0.21b | 2.98 ± 0.22b |
| LDL-C 2 | 5.60 ± 0.40c | 5.24 ± 0.61abc | 5.42 ± 0.45bc | 4.83 ± 0.42ab | 4.99 ± 0.47abc | 4.73 ± 0.58a |
| GOT 2 | 1.35 ± 0.16c | 0.67 ± 0.06a | 0.75 ± 0.08a | 1.05 ± 0.07b | 1.08 ± 0.11b | 1.02 ± 0.11b |
| GPT 2 | 1.66 ± 0.21b | 1.50 ± 0.15ab | 1.30 ± 0.17a | 1.37 ± 0.14a | 1.32 ± 0.11a | 1.89 ± 0.21c |
| hepatopancreas biochemical indices | ||||||
| ATGL 2 | 120.27 ± 13.39a | 160.70 ± 14.42b | 180.99 ± 19.98bc | 172.43 ± 9.06b | 197.69 ± 14.27 cd | 204.79 ± 28.43d |
| HSL 2 | 121.45 ± 7.97a | 129.93 ± 15.97a | 210.20 ± 18.62b | 128.23 ± 8.69a | 124.60 ± 10.14a | 128.25 ± 15.23a |
| FAS 2 | 18.46 ± 0.63a | 21.08 ± 0.58b | 23.37 ± 1.47c | 18.83 ± 1.64a | 18.51 ± 1.02a | 18.16 ± 1.27a |
| Regression | ||||||
| YPWG = −31.219 x2 + 64.526 × + 93.23 | X = 1.03 | R2 = 0.8085 | P = 0.084 | |||
Values are means ± SD for three replicate groups, with 25 fish in each group, and mean values within the same row with different superscripts are significantly different (P < 0.05). 2 Values are means ± SD (n = 6), and mean values within the same row with different superscripts are significantly different (P < 0.05). IBW: initial body weight (g/fish); FBW: final body weight (g/fish); PWG: percent weight gain (%); FI: feed intake (g/fish); FE: feed efficiency (%); CF: condition factor (g/cm3); Flesh rate (%); slaughter rate (%); TG: total triglyceride (mmol/L); TC: total cholesterol (mmol/L); HDL–C: high density lipoprotein cholesterol (mmol/L); LDL-C: low density lipoprotein cholesterol (mmol/L); GOT: glutamic oxaloacetic transaminase (U/L); GPT: glutamic pyruvic transaminase (U/L); FAS: fatty acid synthase (U/g tissue); ATGL: adipose triglyceride lipase (U/g tissue); HSL: hormone-sensitive lipase (U/g protein).
Hepatopancreas health is important for maintaining fish growth and development. Serum GOT and GPT activities, hepatopancreas H & E staining and lipid droplet area reflect hepatopancreas health, and reduction in GOT and GPT activities and lipid droplet area indicated an improvement in hepatopancreas health (Zhu et al., 2022). As seen in Table 1, compared to the control group, the ratio of 0.92 significantly reduced serum GOT and GPT activities in grass carp (P < 0.05). Fig. 1A observes that that the ratios of 0.03 and 2.15 have a large number of vacuoles and most of the nuclei are squeezed to one side of the cells compared to the ratio of 0.92, and the ratio of 2.15 cell-to-cell gaps is unclear. Fig. 1B observed red (lipid droplets) in the field of view and the number of lipid droplets with ALA/LNA ratio of 0.92 was less than those with ALA/LNA ratios of 0.03 and 2.15. Fig. 1D showed that the hepatopancreas lipid droplet area was lowest at the ALA/LNA ratio of 0.92 (P < 0.05). All of these results indicated that dietary appropriate ALA/LNA ratio is beneficial to fish hepatopancreas health.
Fig. 1.
Effects of ALA/LNA ratios on histological sections of hepatopancreas and muscle in grass carp (n = 3). (A) Hematoxylin-eosin (H&E) staining of hepatopancreas, scale bar = 10 µm (×400), vacuolation (★), migration of nucleus (black arrows). (B) Oil red O staining of hepatopancreas, scale bar = 50 µm (×200) red part is lipid droplets (black arrows); (C) Oil red O staining of muscle, scale bar = 100 µm (×200), red part is lipid droplets (black arrows); (D) The quantitative graph of oil red O staining. Values are means ± SD. Mean values within the same row with different superscripts are significantly different (P < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Flesh quality
Besides growth performance, consumers are concerned about flesh quality. pH value, shear force and cooking loss as technological properties can affect flesh quality (Xiong, Sun, Zeng, & Xie, 2014). Shear force reflects the hardness of the meat, and the higher shear force indicates the harder meat. During cooking, the lost components are water, some sarcoplasmic proteins and intramuscular fat-soluble collagen. The smaller the cooking loss, the better is considered the flesh quality. As shown in Table 2, compared to control group, the ratios of 0.47 to 1.33 significantly increased the pH24h value of grass carp muscle (P < 0.05), the ratio of 0.92 increased muscle shear force (P < 0.05), and the ratios of 0.47 and 0.92 significantly decreased the cooking loss of muscle (P < 0.05). These results suggested that dietary appropriate ALA/LNA ratio enhanced the technical properties of fish. Changes in fish pH value after death was associated with the accumulation of lactic acid (Wang et al., 2015). As displayed in Table 2, the muscle lactate content of grass carp reached its lowest value at a ratio of 0.47 (P < 0.05). Therefore, dietary ALA/LNA ratios-mediated pH24h value increase in muscle might be associated with a reduction in lactate content. Furthermore, protein in meat break down into alkaline substances such as ammonia and amino acids, making meat alkaline and leading to an increase in pH (Huang et al., 2023). Therefore, we speculated that dietary appropriate ALA/LNA ratio might better alleviate the protein decomposition rate, improve antioxidant capacity, and reduce muscle pH value.
Table 2.
Effects of dietary ALA/LNA ratios on muscle nutrient components, physicochemical property in sub-adult grass carp.
| ALA/LNA ratios | 0.03 | 0.47 | 0.92 | 1.33 | 1.69 | 2.15 |
|---|---|---|---|---|---|---|
| Muscle nutrition components | ||||||
| Water | 77.93 ± 0.35c | 78.09 ± 0.9c | 72.10 ± 0.77a | 75.46 ± 0.44b | 75.47 ± 0.69b | 75.35 ± 1.14b |
| Crude lipid | 2.02 ± 0.10a | 2.01 ± 0.09a | 3.80 ± 0.19c | 2.35 ± 0.15b | 2.43 ± 0.11b | 2.45 ± 0.11b |
| Crude protein | 18.89 ± 0.48a | 19.10 ± 0.15ab | 21.39 ± 1.04d | 20.21 ± 0.76c | 20.07 ± 1.03bc | 20.47 ± 1.18 cd |
| Muscle physicochemical property | ||||||
| pH24h | 6.37 ± 0.04a | 6.43 ± 0.05bc | 6.54 ± 0.03d | 6.43 ± 0.03c | 6.39 ± 0.02ab | 6.36 ± 0.02a |
| Lactate | 1.52 ± 0.09c | 1.32 ± 0.12a | 1.43 ± 0.05abc | 1.40 ± 0.09ab | 1.41 ± 0.10abc | 1.48 ± 0.07bc |
| Shear force | 10.87 ± 1.03a | 13.68 ± 2.22b | 10.73 ± 1.48a | 8.82 ± 0.43a | 10.81 ± 2.70a | 10.50 ± 1.73a |
| Cooking loss | 16.25 ± 0.84c | 13.73 ± 1.03a | 14.65 ± 0.60ab | 15.53 ± 1.33bc | 16.27 ± 1.15c | 16.83 ± 1.33c |
Values are means ± SD (n = 6). Mean values within the same row with different superscripts are significantly different (P < 0.05). water (%); crude lipid (%); crude protein (%); lactate content (mmol/g protein); shear force (N); cooking loss (%).
In addition to technological properties, crude lipid and protein contents as important chemical parameters can also reflect flesh quality (Xiong, Sun, Zeng, & Xie, 2014). Suitable water can make the fish tender and juicy, protein content can affect the hardness of the meat and fat content has an important effect on the sensory quality of the fish. From Table 2, we know that when the ALA/LNA ratio was 0.92, the water content of grass carp muscle was significantly lower than the other groups (P < 0.05), and the protein and crude lipid content were significantly higher than the other groups (P < 0.05). It showed that dietary appropriate ALA/LNA ratio improved the chemical properties of fish. In addition, lipid content can be reflected by lipid droplet area. The results of muscle oil red O staining and quantification (Fig. 1C and Fig. 1E) informed that the 0.92 ratio of grass carp muscle had the largest lipid droplet area (P < 0.05). It might be related to PPARγ pathway. PPARγ is a key molecule in lipid accumulation (Marion Letellier, Savoye, & Ghosh, 2016). From Fig. 2B-C, we know that when the ALA/LNA ratio was 0.92, the mRNA and protein levels of PPARγ were significantly higher than other groups in muscle of grass carp (P < 0.05). Further analysis showed a positive correlation between crude lipid content and PPARγ protein level (r = +0.719, P = 0.108), which supports our hypothesis.
Fig. 2.
Effect of ALA/LNA ratios on fatty acid metabolism in muscle of grass carp. (A) Enzymes activities related to fatty acid synthesis; (B) The heat map of gene mRNA levels; (C) The protein levels of SREBP1, PPARα and PPARγ. Values are means ± SD (n = 6). Mean values within the same row with different superscripts are significantly different (P < 0.05). FAS: fatty acid synthase (U/g tissue); Fad: fatty acid desaturase (U/g tissue); Elovl: fatty acid elongase (U/g tissue).
Lipid deposition in fish muscle is mainly involved in adipogenesis, lipid transport, lipid uptake and lipid storage (Yuan et al., 2016). Therefore, we continued to explore the effect of dietary ALA/LNA ratios on the mentioned processes in grass carp.
3.3. Liver lipid metabolism and lipid transport
Liver is the main site of fatty acid synthesis and lipolysis in fish. FAS is main enzyme of fatty acid synthesis, while ATGL and HSL are main enzymes of lipolysis (Guillou, Martin, & Pineau, 2008). Table 1 showed that the hepatopancreas HSL and FAS activities at the ratio of 0.92 were significantly higher than other groups (P < 0.05). It indicated that dietary appropriate ALA/LNA ratio improved lipid synthesis and lipolysis in fish. However, the hepatopancreas ATGL activity increased significantly with the increase of ALA/LNA ratio (P < 0.05) (Table 1). We speculated that the ratio of 2.15 might be present with fatty liver symptoms and that the hepatopancreas secreted more ATGL to specifically hydrolyze triglycerides to maintain homeostasis.
Lipid synthesized in liver can be transported to the peripheral tissues through the blood. Elevated serum TG and TC contents indicate active fish metabolism (Yuan et al., 2016). Serum HDL-C content was negatively associated with the development of coronary artery disease, while LDL-C content was positively associated with it. As shown in Table 1, when the ALA/LNA ratio was 0.92, serum TC TG and HDL-C contents were reached maximum value (P < 0.05), LDL-C content decreased significantly with the increase of ALA/LNA ratio (P < 0.05). This result indicated that dietary appropriate ALA/LNA ratio promoted the metabolism and blood lipid transport in fish. Blood absorption of fatty acids into skeletal muscle depends on the regulation of transporters (e.g. FAT/CD36 and FABP1), of which FABP1 is present in skeletal muscle and can be involved in the uptake and transport of long-chain fatty acids (C. L. Li, Zhang, Qiu, Deng, & Wang, 2022). Fig. 2B showed that compared with control group, the ratio of 0.92–1.69 significantly increased the muscle FAT/CD36 mRNA level (P < 0.05) and the ratio of 1.33 significantly increased the FABP1 mRNA level of muscle (P < 0.05), which indicated that dietary appropriate ALA/LNA ratio enhanced fatty acids cellular uptake in fish.
Lipid can reach muscle and affects lipid metabolism in animal muscle. Therefore, we continued to investigate the influence of dietary ALA/LNA ratios on muscle fatty acid metabolism.
3.4. Muscle lipid metabolism
Fatty acids can be divided into saturated and unsaturated fatty acids according to the degree of saturation. UFA has benefits such as increased nerve cell formation, healthy vision, and enhanced immunity. OA (18:1n9), EPA (20:5n3) and DHA (22:6n3) may reduce inflammation and lower the risk of cardiovascular disease and cancer (Li, Zhang, Qiu, Deng, & Wang, 2022). A lower ratio of n6/n3 contributes to anti-inflammatory and anti-cancer, and ΣMUFA/ΣSFA is beneficial to human health. The World Health Organization and the Food and Agriculture Organization of the United Nations (FAO) recommend ΣPUFA/ΣSFA > 0.4 as a standard for desirable fatty acids (FAO, 2022). Table 3 showed that compared to the control group, the ratio of 0.92–2.15 significantly increased muscle OA, ΣMUFA, ΣUFA and ΣMUFA/ΣSFA contents (P < 0.05), the ratio of 0.47–2.15 significantly increased muscle EPA content (P < 0.05), the ratio of 0.47 significantly increased DHA and ΣPUFA contents (P < 0.05), and the ratio of 0.47–2.15 significantly decreased muscle n6/n3 ratio (P < 0.05), and the ΣPUFA/ΣSFA values of grass carp muscle were all > 0.4. These results suggested that dietary appropriate ALA/LNA ratio improved healthcare value of fish. The increase in ΣMUFA content might be associated with enzymes that regulate its synthesis. The synthesis of MUFA requires FAS, ACC and SCD1 (C. L. Li, Zhang, Qiu, Deng, & Wang, 2022). As displayed in Fig. 2 A-B, compared to the control group, the ratio of 0.92–2.15 significantly increased the mRNA levels of FAS, ACC and SCD1 in muscle (P < 0.05), the ratio of 0.92 and 1.33 significantly improved muscle FAS activity (P < 0.05). Correlation analyses showed that ΣMUFA content was positively correlated with FAS and ACC mRNA levels, which supports our hypothesis. The increase in ΣPUFA content might be linked to Elovl and Fad. The synthesis of PUFA requires Elovl and Fad (Xie et al., 2021). As shown in Fig. 2 A-B, compared with the control group, the ratio of 0.47–2.15 significantly increased the mRNA levels and activity of Fad in muscle (P < 0.05), the ratio of 0.47–1.33 significantly increased the activity of Elovl in muscle (P < 0.05), the ratio of 0.92 and 1.33 significantly improved the mRNA levels of Elovl2 in muscle (P < 0.05). Therefore, we speculated that PUFA synthesis induced by dietary ALA/LNA ratios might related to expressions and activities of Elovl and Fad. In conclusion, dietary appropriate ALA/LNA ratio promoted fatty acid synthesis in fish muscle. The influences of ALA/LNA ratios on FAS, ACC, SCD1, Elovl and Fad might be associated with LXRα/SREBP-1 signaling pathway. LXRα/SREBP-1 signaling pathway is crucial for fatty acid synthesis by regulating FAS, ACC, SCD1, Fad, and Elovl (C. L. Li et al., 2022, Xie et al., 2021). Fig. 2B-C showed that compared with control group, the ratio of 0.47 and 0.92 significantly increased the mRNA levels of SREBP-1 and LXRα in muscle (P < 0.05) and the ratio of 0.92–1.69 significantly increased the SREBP-1 protein level of muscle (P < 0.05). Correlation analyses showed that Elovl2, Fad, FAS, and ACC mRNA levels were positively correlated with SREBP-1 protein level, which supports our hypothesis.
Table 3.
Effects of dietary ALA/LNA ratios fatty acid (FA) profile (% of total FA methyl esters) in muscle of sub-adult grass carp.
| ALA/LNA ratios | 0.03 | 0.47 | 0.92 | 1.33 | 1.69 | 2.15 |
|---|---|---|---|---|---|---|
| C14:1 | 0.107 ± 0.003a | 0.111 ± 0.002a | 0.122 ± 0.003b | 0.112 ± 0.002a | 0.111 ± 0.009a | 0.111 ± 0.005a |
| C15:0 | 0.098 ± 0.004b | 0.096 ± 0.100b | 0.094 ± 0.001a | 0.104 ± 0.001c | 0.105 ± 0.001c | 0.105 ± 0.001c |
| C16:0 | 21.58 ± 0.38c | 21.57 ± 0.15c | 20.41 ± 0.03ab | 21.20 ± 0.06bc | 19.72 ± 0.98a | 21.17 ± 1.34bc |
| C16:1 | 9.09 ± 0.05a | 9.11 ± 0.04a | 9.74 ± 0.02c | 9.59 ± 0.03bc | 9.38 ± 0.41abc | 9.30 ± 0.68ab |
| C18:0 | 4.59 ± 0.13b | 4.85 ± 0.05c | 4.61 ± 0.02b | 4.51 ± 0.03b | 4.51 ± 0.23b | 4.29 ± 0.03a |
| C18:1n9 (OA) | 34.86 ± 0.25a | 33.64 ± 0.12a | 36.03 ± 0.11b | 38.46 ± 0.09b | 38.74 ± 2.71b | 38.98 ± 0.06b |
| C18:2n6 (LNA) | 11.50 ± 0.11c | 8.57 ± 0.03b | 7.42 ± 0.01a | 7.61 ± 0.05a | 7.46 ± 1.38a | 7.78 ± 0.06a |
| C18:3n3 (ALA) | 0.058 ± 0.008a | 0.056 ± 0.001a | 0.075 ± 0.001c | 0.075 ± 0.001bc | 0.073 ± 0.002bc | 0.070 ± 0.005b |
| C18:3n6 | 0.147 ± 0.004d | 0.124 ± 0.004c | 0.106 ± 0.001a | 0.115 ± 0.001b | 0.146 ± 0.015d | 0.148 ± 0.001d |
| C20:0 | 0.091 ± 0.006b | 0.073 ± 0.003a | 0.075 ± 0.001a | 0.079 ± 0.001a | 0.092 ± 0.014b | 0.094 ± 0.004b |
| C20:1n9 | 0.06 ± 0.001a | 0.21 ± 0.002b | 0.34 ± 0.002c | 0.41 ± 0.002d | 0.46 ± 0.002f | 0.459 ± 0.001e |
| C20:2 | 0.77 ± 0.005e | 0.71 ± 0.009d | 0.59 ± 0.000a | 0.60 ± 0.004b | 0.61 ± 0.005b | 0.64 ± 0.005c |
| C20:3n3 | 0.06 ± 0.002a | 0.20 ± 0.005b | 0.26 ± 0.002c | 0.29 ± 0.004d | 0.32 ± 0.003e | 0.33 ± 0.005f |
| C20:3n6 | 1.07 ± 0.005c | 1.08 ± 0.004c | 0.75 ± 0.002b | 0.73 ± 0.002ab | 0.70 ± 0.072a | 0.73 ± 0.002ab |
| C20:5n3 (EPA) | 0.81 ± 0.01a | 1.73 ± 0.02b | 1.66 ± 0.01b | 1.67 ± 0.01b | 1.64 ± 0.36b | 1.77 ± 0.01b |
| C22:0 | 1.51 ± 0.006b | 1.93 ± 0.007c | 1.37 ± 0.003a | 1.35 ± 0.004a | 1.35 ± 0.251a | 1.37 ± 0.003a |
| C22:1n9 | 0.19 ± 0.003c | 0.15 ± 0.002b | 0.11 ± 0.001a | 0.15 ± 0.001b | 0.29 ± 0.063d | 0.30 ± 0.002d |
| C22:6n3 (DHA) | 5.94 ± 0.34a | 8.96 ± 0.36b | 6.69 ± 0.03a | 6.46 ± 0.03a | 6.66 ± 1.64a | 6.50 ± 0.02a |
| C23:0 | 2.36 ± 0.01b | 2.59 ± 0.01c | 1.52 ± 0.01a | 1.50 ± 0.01a | 1.54 ± 0.36a | 1.57 ± 0.01a |
| ΣSFA | 35.13 ± 0.16c | 35.09 ± 0.22c | 32.80 ± 0.05ab | 33.36 ± 0.05b | 32.36 ± 1.10a | 32.64 ± 0.99ab |
| ΣMUFA | 44.47 ± 0.26a | 43.61 ± 0.51a | 49.50 ± 0.09b | 48.90 ± 0.09b | 49.15 ± 2.78b | 49.62 ± 0.04b |
| ΣPUFA | 20.41 ± 0.38b | 21.48 ± 0.34c | 17.70 ± 0.11a | 17.75 ± 0.13a | 17.81 ± 2.11a | 18.12 ± 0.09a |
| ΣUFA | 64.87 ± 0.16a | 65.09 ± 0.41a | 67.20 ± 0.05b | 66.64 ± 0.05b | 66.47 ± 2.29b | 66.85 ± 1.46b |
| Σn3PUFA | 6.87 ± 0.35a | 10.94 ± 0.36c | 8.69 ± 0.04b | 8.49 ± 0.03b | 7.98 ± 2.93ab | 8.67 ± 0.03b |
| Σn6PUFA | 12.72 ± 0.11c | 9.78 ± 0.04b | 8.28 ± 0.02a | 8.46 ± 0.05a | 8.68 ± 1.01a | 8.65 ± 0.06a |
| ALA/LNA | 0.005 ± 0.001a | 0.007 ± 0.000b | 0.010 ± 0.000d | 0.0010 ± 0.000d | 0.008 ± 0.001c | 0.009 ± 0.001c |
| n6/n3 | 1.86 ± 0.01c | 0.89 ± 0.03a | 0.95 ± 0.00a | 1.00 ± 0.01ab | 1.19 ± 0.37b | 1.00 ± 0.01ab |
| ΣMUFA/ΣSFA | 1.27 ± 0.01a | 1.24 ± 0.01a | 1.51 ± 0.00b | 1.47 ± 0.01b | 1.52 ± 0.12b | 1.52 ± 0.04b |
| ΣPUFA/ΣSFA | 0.58 ± 0.01bc | 0.61 ± 0.01c | 0.54 ± 0.00ab | 0.53 ± 0.00a | 0.55 ± 0.08ab | 0.56 ± 0.02ab |
Values are means ± SD (n = 6). Mean values within the same row with different superscripts are significantly different (P < 0.05). ΣSFA, total saturated fatty acids; ΣUFA, total unsaturated fatty acids; ΣMUFA, total monounsaturated fatty acids; ΣPUFA, total polyunsaturated fatty acids; OA: oleic acid; ALA: alpha-linolenic acid; LNA: linolenic acid; EPA: eicosapentaenoic acid; DHA: docosahexenoic acid.
Besides fatty acid synthesis, muscle also has fatty acid catabolism. Fatty acid catabolism requires CPT1 and ACO, CPT1 acts on straight-chain fatty acids with <20 carbon atoms and ACO acts on very long-chain fatty acids (Guillou, Martin, & Pineau, 2008). Fig. 2B showed that compared with control group, the ratio of 0.92–2.15 significantly increased the mRNA levels of CPT1a and ACO1a in muscle (P < 0.05) and the ratio of 0.47–2.15 significantly increased the CPT1b mRNA level of muscle (P < 0.05), which indicated that appropriate ALA/LNA ratio enhanced fatty acid catabolism in fish muscle. It might be associated with PPARα signaling pathway. PPARα can promote oxidation of fatty acids by regulating transcription of ACO and CPT1 (Guillou, Martin, & Pineau, 2008). As displayed in Fig. 2B-C, when ALA/LNA ratio was 1.33, the mRNA and protein levels of PPARα in muscle were higher than other groups (P < 0.05). Correlation analyses showed that ACO1a and CPT1b mRNA levels were positively correlated with PPARα protein level, which supports our hypothesis.
There were two interesting results from this study. Firstly, ALA/LNA ratios had no effect on Elovl5 mRNA level in muscle, which might be related to the function of Elovl2 and Elovl5. The ability of grass carp Elovl2 to extend C18 and C20 PUFAs overlaps with that of Elovl5, and in addition, Elovl2 extends C22 PUFAs, especially to generate 22:5n-3 to 24:5n-3 (Marrero et al., 2022). Secondly, muscle ALA/LNA ratios were inconsistent with dietary ALA/LNA ratios, which was inconsistent with muscle fatty acid composition reflecting dietary fatty acid composition (Nayak, Giri, Pradhan, Samanta, & Saha, 2020). It might be related to ALA metabolism. Study found that ALA was mainly used for oxidation in muscle tissue, part of which was converted into long-chain fatty acids (EPA, DPA, DHA), and a small part was accumulated in muscle in the form of ALA (Punia, Sandhu, Siroha, & Dhull, 2019).
In conclusion, dietary appropriate ALA/LNA ratio promoted anabolism and catabolism of fatty acid in fish muscle, and anabolism might be stronger than catabolism due to the increased crude lipid content in muscle. Fatty acid oxidation affected glucose metabolism. Therefore, we continued to study effect of ALA/LNA ratios on glucose metabolism.
3.5. Muscle glucose metabolism
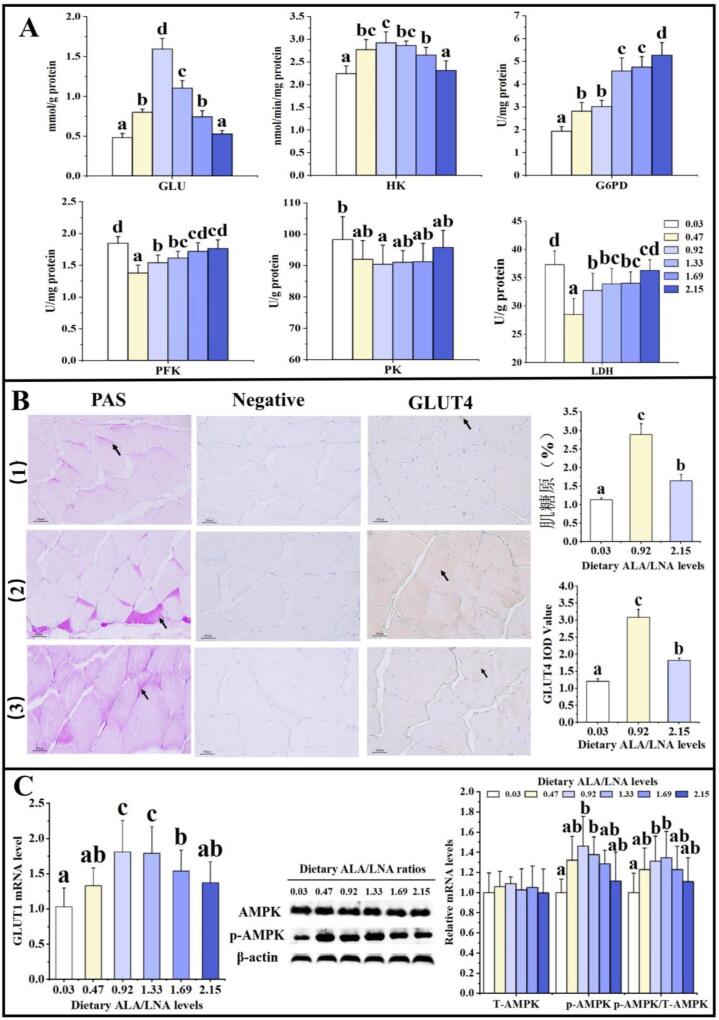
Glucose is a source of energy for animals. Fig. 3A showed that the ratio of 0.47–1.69 significantly increased muscle glucose content (P < 0.05). It might be associated with glucose uptake. Animal muscles need to rely on the transmembrane transport function of glucose transport proteins (GLUT1 and GLUT4) for glucose uptake (Injarabian, Devin, Ransac, & Marteyn, 2020). As shown in Fig. 3B-C, the ratio of 0.92 significantly increased the mRNA level of GLUT1 and IOD value of GLUT4 in muscle (P < 0.05), which indicated that dietary appropriate ALA/LNA ratio promoted glucose uptake in fish.
Fig. 3.
Effects of ALA/LNA ratios on glucose metabolism in muscle of grass carp. (A) Enzyme activities and content of glucose metabolism indexes (n = 6); (B) PAS staining and Immunohistochemistry of muscle, (1): ALA/LNA = 0.03, (2): ALA/LNA = 0.92, (3): ALA/LNA = 2.15, scale bar = 50 µm (×200) (n = 3), purple part is glycogen (black arrows), brown-yellow part is GLUT4 (black arrows); (C) The mRNA level of GLUT1 and the protein levels of T-AMPK, p-AMPK, and p-AMPK/ T-AMPK (n = 6). Values are means ± SD. Mean values within the same row with different superscripts are significantly different (P < 0.05). GLU: glucose (mmol/g protein); HK: hexokinase (nmol/min/mg protein); G6PD: glucose-6-phosphate dehydrogenase (U/mg protein); PFK: phosphofructokinase (U/mg protein); PK: pyruvate kinase (U/g protein); LDH: lactate dehydrogenase (U/g protein). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Glucose can reach muscle and affects glucose metabolism in animal muscle. Glucose is firstly phosphorylated by HK to produce glucose 6 phosphate (G6P) (Wang et al., 2021). Fig. 3A showed that the ratio of 0.47–1.69 significantly improved HK activity in muscle (P < 0.05). There are three ways of G6P metabolism. Firstly, G6P can be converted into muscle glycogen and stored (Injarabian, Devin, Ransac, & Marteyn, 2020). High glycogen levels at slaughter could prevent dark-firm-dry syndrome and improve meat quality (Pethick, Rowe, & Tudor, 1995). As displayed in Fig. 3B, the ratio of 0.92 had higher glycogen content in muscle than other groups (P < 0.05). These findings suggested that dietary optimal ALA/LNA ratio increased muscle glycogen content in fish. Secondly, G6P can enter pentose phosphate pathway, and G6PD is the rate-limiting enzyme (Wang et al., 2021). Fig. 3A showed that the enzymatic activity of G6PD in muscle increased significantly with the increase of ALA/LNA ratio (P < 0.05), which suggested that dietary ALA/LNA ratios increased pentose phosphate pathway in fish. Thirdly, G6P can produce pyruvate by glycolysis, which produces lactic acid under the action of LDH (Injarabian, Devin, Ransac, & Marteyn, 2020). Fig. 3A showed that the ratio of 0.47–1.69 significantly reduced LDH activity in muscle (P < 0.05). It might be associated with glycolysis-related enzymes. The two rate-limiting enzymes of glycolysis are PFK and PK (Wang et al., 2021). As shown in Fig. 3A, when the ALA/LNA ratio were 0.47–1.33, grass carp had lower PFK activity than control group (P < 0.05), the ratio of 0.92 significantly reduced PK activity in muscle (P < 0.05). Further analysis showed a positive correlation of lactate content with PFK, PK and LDH activities, which supports our hypothesis. Therefore, dietary appropriate ALA/LNA ratio inhibited anaerobic metabolism in fish. Collectively, dietary appropriate ALA/LNA ratio increased glycogen content and pentose phosphate pathway, inhibited anaerobic metabolism.
The influences of dietary ALA/LNA ratios on glucose uptake and utilization might be associated with AMPK signaling pathway. AMPK can stimulate glucose uptake and utilization in skeletal muscle of fish (Magnoni, Vraskou, Palstra, & Planas, 2012). Fig. 3C discovered that the ratio of 0.47 and 0.92 improved protein levels of p-AMPK and p-AMPK/T-AMPK in muscle (P < 0.05). Correlation analyses showed a positive correlation between HK activity, GLUT1 mRNA level and p-AMPK protein level, and a negative correlation between PFK, PK, LDH activities and p-AMPK protein level, which supports our hypothesis.
3.6. Adverse effects of excess ALA/LNA ratio on fish
This study found that excess ALA/LNA ratio (2.15) had an adverse effect on growth performance (FI, PWG, FE, CF) compared to optimal ALA/LNA ratio. The above result might be related to the digestive and absorptive capacity of the intestine. Study on juvenile grass carp in our laboratory showed that high ALA/LNA ratio reduced activities and mRNA levels of digestive enzymes and brush margin enzymes in intestine (Zeng et al., 2016). Excess ALA/LNA ratio (2.15) also had adverse effects on most indexes of lipid metabolism and glucose metabolism (except UFA, OA, SFA, MUFA, EPA contents and serum TC content). The downregulation of lipid metabolism and glucose metabolism mediated by excess ALA/LNA ratio might be associated with hormone levels. Research showed that high intake of ALA decreased plasma adiponectin level in rat (Zhang et al., 2016). Animal tissues such as skeletal muscle had worse lipid metabolism due to reduced adiponectin level (Lee & Shao, 2012). Research found that ALA at high dose inhibited prostaglandin E2 (PGE2) production in synovial explants from horses (Munsterman, Bertone, Zachos, & Weisbrode, 2005), and decreased PGE2 level down-regulated GLUT1 mRNA level and glucose uptake in primary human adipose stromal cells (Docanto, Ham, Corbould, & Brown, 2015). However, it still requires further investigation.
3.7. Proper ALA/LNA ratio
In our experiment, dietary optimal ALA/LNA ratio of sub-adult grass carp was 1.03 according to the quadratic regression analysis of PWG, which was slightly lower than that of juvenile grass carp (1.08) (Zeng et al., 2016). It might be associated with faster growth rate of juvenile grass carp compared to sub-adult grass carp. Dietary optimal ALA/LNA ratio determined by muscle glucose and UFA contents was 0.92 and 0.88, respectively. Dietary optimal ALA/LNA ratio determined by UFA content was lower than that determined by growth performance. UFA is classified as MUFA and PUFA according to the degree of saturation. High linolenic acid content reduced MUFA content of longissimus lumborum in rabbits (Du, Wang, Wang, Ma, & Li, 2013). Study found that freshwater fish could use ALA and LNA to synthesize PUFA, which requires Elovl and Fad, thus there was metabolic inhibition of ALA and LNA, and the affinity of ALA for Elovl and Fad was higher than that of LNA (Nayak, Giri, Pradhan, Samanta, & Saha, 2020). Therefore, dietary optimal ALA/LNA ratio was lower than that of growth performance in order to maintain high UFA content. However, this still needs further investigation.
4. Conclusions
This study investigated the effect of dietary ALA/LNA ratio on lipid metabolism and glucose metabolism, as well as meat quality in fish, and there were three novel findings. (1) Optimal ALA/LNA ratio promoted muscle chemical properties (lipid, protein), technological properties (pH, shear force) and healthcare properties (EPA, DHA, and OA contents) in fish. (2) Optimal ALA/LNA ratio increased crude lipid content might be related to fatty acid anabolism and catabolism, and anabolism might be stronger than catabolism. Meanwhile, optimal ALA/LNA ratio enhanced fatty acid metabolism and catabolism, which was linked to related signaling pathways (LXRα/SREBP-1, PPARα, PPARγ) in fish muscle. (3) Optimal ALA/LNA ratio enhanced muscle glucose uptake and utilization by promoting muscle glycogen synthesis and pentose phosphate pathway, and inhibiting anaerobic metabolism in fish, which were associated with AMPK signaling pathway. Based on PWG, muscle glucose and UFA contents, dietary optimal ALA/LNA ratio was 1.03, 0.92, 0.88, respectively.
CRediT authorship contribution statement
Chi-Bei Yao: Conceptualization, Methodology, Data curation, Writing – original draft. Lin Feng: Supervision, Writing – review & editing, Project administration. Pei Wu: Methodology, Writing – review & editing. Yang Liu: Writing – review & editing, Project administration. Jun Jiang: Methodology, Writing – review & editing. Lu Zhang: Resources, Writing – review & editing. Hai-Feng Mi: Resources, Writing – review & editing. Xiao-Qiu Zhou: Supervision, Project administration, Funding acquisition. Wei-Dan Jiang: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was financially supported by the earmarked fund for CARS (CARS-45), Outstanding Youth Science Foundation of Sichuan Province (2020JDJQ0043), National Key R&D Program of China (2018YFD0900400 and 2019YFD0900200), and National Natural Science Foundation of China for Outstanding Youth Science Foundation (31922086). The authors would like to thank the personnel of these teams for their kind assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.100752.
Contributor Information
Xiao-Qiu Zhou, Email: zhouxq@sicau.edu.cn.
Wei-Dan Jiang, Email: WDJiang@sicau.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- Bendinelli P., Piccoletti R., Maroni P. Leptin rapidly activates PPARs in C2C12 muscle cells. Biochemical and Biophysical Research Communications. 2005;332(3):719–725. doi: 10.1016/j.bbrc.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Berge G.M., Witten P.E., Baeverfjord G., Vegusdal A., Wadsworth S., Ruyter B. Diets with different n-6/n-3 fatty acid ratio in diets for juvenile Atlantic salmon, effects on growth, body composition, bone development and eicosanoid production. Aquaculture. 2009;296(3–4):299–308. doi: 10.1016/j.aquaculture.2009.08.029. [DOI] [Google Scholar]
- Bouskila M., Hirshman M.F., Jensen J., Goodyear L.J., Sakamoto K. Insulin promotes glycogen synthesis in the absence of GSK3 phosphorylation in skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism. 2008;294(1):E28–E35. doi: 10.1152/ajpendo.00481.2007. [DOI] [PubMed] [Google Scholar]
- Brinker A., Reiter R. Fish meal replacement by plant protein substitution and guar gum addition in trout feed, Part I: Effects on feed utilization and fish quality. Aquaculture. 2011;310(3–4):350–360. doi: 10.1016/j.aquaculture.2010.09.041. [DOI] [Google Scholar]
- Docanto M., Ham S., Corbould A., Brown K. Obesity-associated inflammatory cytokines and prostaglandin E2 stimulate glucose transporter mRNA expression and glucose uptake in primary human adipose stromal cells. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2015;35 doi: 10.1089/jir.2014.0194. [DOI] [PubMed] [Google Scholar]
- Du H.-T., Wang C.-Y., Wang X.-P., Ma M.-W., Li F.-C. The effects of dietary α-linolenic acid on growth performance, meat quality, fatty acid composition and liver relative enzyme mRNA expression of growing meat rabbits. Journal of Animal and Feed Sciences. 2013;22(2):122–129. doi: 10.22358/jafs/66002/2013. [DOI] [Google Scholar]
- FAO. (2022). The State of World Fisheries and Aquaculture 2022. Towards Blue. Transformation. Rome. https://www.fao.org/3/cc0461en/cc0461en.pdf.
- Glencross B.D. Exploring the nutritional demand for essential fatty acids by aquaculture species. Reviews in Aquaculture. 2009;1(2):71–124. doi: 10.1111/j.1753-5131.2009.01006.x. [DOI] [Google Scholar]
- Guillou H., Martin P.G.P., Pineau T. Transcriptional regulation of hepatic fatty acid metabolism. Sub-cellular Biochemistry. 2008;49:3–47. doi: 10.1007/978-1-4020-8831-5_1. [DOI] [PubMed] [Google Scholar]
- Huang Y.S., Lin Z.D., Rong H., Hao M.L., Zhu D.S., Li S.K., et al. Effects of conjugated linoleic acid on growth, body composition, antioxidant status, lipid metabolism and immunity parameters of juvenile Chu’s croaker, Nibea coibor. Aquaculture Research. 2018;49(1):546–556. doi: 10.1111/are.13486. [DOI] [Google Scholar]
- Huang Y., Zhou Y., Liu Y., Wan J., Hu P., Liu L., et al. Effects of tea branch liquid smoke on oxidation and structure of myofibrillar protein derived from pork tenderloin during curing. Food Chemistry: X. 2023;17 doi: 10.1016/j.fochx.2022.100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Injarabian L., Devin A., Ransac S., Marteyn B.S. Neutrophil metabolic shift during their lifecycle: impact on their survival and activation. International Journal of Molecular Sciences. 2020;21(1) doi: 10.3390/ijms21010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S.K., Liu X., Samagh S.P., Lovett D.H., Bodine S.C., Kim H.T., et al. mTOR regulates fatty infiltration through SREBP-1 and PPARγ after a combined massive rotator cuff tear and suprascapular nerve injury in rats. Journal Orthopaedic Research. 2013;31(5):724–730. doi: 10.1002/jor.22254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan T., Caballero-Solares A., Taylor R.G., Rise M.L., Parrish C.C. Effect of plant-based diets with varying ratios of omega 6 to omega 3 fatty acids on growth performance, tissue composition, fatty acid biosynthesis and lipid-related gene expression in Atlantic salmon (Salmo solar) Comparative Biochemistry and Physiology D-Genomics & Proteomics. 2019;30:290–304. doi: 10.1016/j.cbd.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Lai M.-C., Teng T.-H., Yang C. The natural PPAR agonist linoleic acid stimulated insulin release in the rat pancreas. Journal of Veterinary Medical Science. 2013;75(11):1449–1454. doi: 10.1292/jvms.13-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Shao J.H. Adiponectin and lipid metabolism in skeletal muscle. Acta Pharmaceutica Sinica B. 2012;2(4):335–340. doi: 10.1016/j.apsb.2012.06.008. [DOI] [Google Scholar]
- Li C.-L., Zhang L.-L., Qiu Z.-D., Deng W.-H., Wang W.-X. Key molecules of fatty acid metabolism in gastric cancer. Biomolecules. 2022;12(5):706. doi: 10.3390/biom12050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima B.T.M., Takahashi N.S., Tabata Y.A., Hattori R.S., Ribeiro C.S., Moreira R.G.J.A. Balanced omega-3 and -6 vegetable oil of Amazonian sacha inchi act as LC-PUFA precursors in rainbow trout juveniles: Effects on growth and fatty acid biosynthesis. Aquaculture. 2019;509:236–245. doi: 10.1016/j.aquaculture.2009.05.004. [DOI] [Google Scholar]
- Ma X., Wang L.-M., Xie D.-Z., Tian X., Zhang Y.-R., Wu L.-M., et al. Effect of dietary linolenic/linoleic acid ratios on growth performance, ovarian steroidogenesis, plasma sex steroid hormone, and tissue fatty acid accumulation in juvenile common carp, Cyprinus carpio. Aquaculture Reports. 2020;18 doi: 10.1016/j.aqrep.2020.100452. [DOI] [Google Scholar]
- Magnoni L.J., Vraskou Y., Palstra A.P., Planas J.V. AMP-activated protein kinase plays an important evolutionary conserved role in the regulation of glucose metabolism in fish skeletal muscle cells. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0031219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion-Letellier R., Savoye G., Ghosh S. Fatty acids, eicosanoids and PPAR gamma. European Journal of Pharmacology. 2016;785:44–49. doi: 10.1016/j.ejphar.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Marrero M., Monroig O., Navarro J.C., Ribes-Navarro A., Perez J.A., Galindo A., et al. Metabolic and molecular evidence for long-chain PUFA biosynthesis capacity in the grass carp Ctenopharyngodon idella. Comparative Biochemistry and Physiology A-Molecular & Integrative Physiology. 2022;270 doi: 10.1016/j.cbpa.2022.111232. [DOI] [PubMed] [Google Scholar]
- Munekata P.E.S., Pateiro M., Lopez-Pedrouso M., Gagaoua M., Lorenzo J.M. Foodomics in meat quality. Current Opinion in Food Science. 2021;38:79–85. doi: 10.1016/j.cofs.2020.10.003. [DOI] [Google Scholar]
- Munsterman A.S., Bertone A.L., Zachos T.A., Weisbrode S.E. Effects of the omega-3 fatty acid, alpha-linolenic acid, on lipopolysaccharide-challenged synovial explants from horses. American Journal of Veterinary Research. 2005;66(9):1503–1508. doi: 10.2460/ajvr.2005.66.1503. [DOI] [PubMed] [Google Scholar]
- Nayak M., Giri S.S., Pradhan A., Samanta M., Saha A. Effects of dietary α-linolenic acid/linoleic acid ratio on growth performance, tissue fatty acid profile, serum metabolites and Δ6 fad and elovl5 gene expression in silver barb (Puntius gonionotus) Journal of the Science of Food and Agriculture. 2020;100(4):1643–1652. doi: 10.1002/jsfa.10177. [DOI] [PubMed] [Google Scholar]
- Nong Q.-Y., Wang L.-Y., Zhou Y.-B., Sun Y., Chen W.-T., Xie J.-T., et al. Low dietary n-6/n-3 PUFA ratio regulates meat quality, reduces triglyceride content, and improves fatty acid composition of meat in heigai pigs. Animals. 2020;10(9) doi: 10.3390/ani10091543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethick, D. W., Rowe, J. B., & Tudor, G. D. (1995). Glycogen metabolism and meat quality. July: 97-102.
- Punia S., Sandhu K.S., Siroha A.K., Dhull S.B. Omega 3-metabolism, absorption, bioavailability and health benefits–A review. PharmaNutrition. 2019;10 doi: 10.1016/j.phanu.2019.100162. [DOI] [Google Scholar]
- Shi X.-C., Sun J., Yang Z., Li X.-X., Ji H., Li Y., et al. Molecular characterization and nutritional regulation of carnitine palmitoyltransferase (CPT) family in grass carp (Ctenopharyngodon idellus) Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology. 2017;203:11–19. doi: 10.1016/j.cbpb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Wang B., Liu Y., Feng L., Jiang W.-D., Kuang S.-Y., Jiang J., et al. Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella) Food Chemistry. 2015;167:91–99. doi: 10.1016/j.foodchem.2014.06.091. [DOI] [PubMed] [Google Scholar]
- Wang C.-C., Si L.-F., Li W.-Y., Zheng J.-L. A functional gene encoding carnitine palmitoyltransferase 1 and its transcriptional and kinetic regulation during fasting in large yellow croaker. Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology. 2019;231:26–33. doi: 10.1016/j.cbpb.2019.01.015. [DOI] [PubMed] [Google Scholar]
- Wang X.-B., Zhang Y.-M., Li Y.-S., Tang M.-Y., Deng Q.-H., Mao J.-D., et al. Estrogen regulates glucose metabolism in cattle neutrophils through autophagy. Frontiers in Veterinary Science. 2021;8 doi: 10.3389/fvets.2021.773514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D.-Z., Chen C.-Y., Dong Y.-W., You C.-H., Wang S.-Q., Monroig O., et al. Regulation of long-chain polyunsaturated fatty acid biosynthesis in teleost fish. Progress in Lipid Research. 2021;82 doi: 10.1016/j.plipres.2021.101095. [DOI] [PubMed] [Google Scholar]
- Xiong Z.-J., Sun D.-W., Zeng X.-A., Xie A.-G. Recent developments of hyperspectral imaging systems and their applications in detecting quality attributes of red meats: A review. Journal of Food Engineering. 2014;132:1–13. doi: 10.1016/j.jfoodeng.2014.02.004. [DOI] [Google Scholar]
- Yuan X.-C., Liang X.-F., Liu L.-W., Fang J.-G., Li J., Li A.-X., et al. Fat deposition pattern and mechanism in response to dietary lipid levels in grass carp, Ctenopharyngodon idellus. Fish Physiology and Biochemistry. 2016;42(6):1557–1569. doi: 10.1007/s10695-016-0240-4. [DOI] [PubMed] [Google Scholar]
- Zeng Y.-Y., Jiang W.-D., Liu Y., Wu P., Zhao J., Jiang J., et al. Optimal dietary alpha-linolenic acid/linoleic acid ratio improved digestive and absorptive capacities and target of rapamycin gene expression of juvenile grass carp (Ctenopharyngodon idellus) Aquaculture Nutrition. 2016;22(6):1251–1266. doi: 10.1111/anu.12337. [DOI] [Google Scholar]
- Zhang J.-N., Wang O., Guo Y.-J., Wang T., Wang S.-Y., Li G.-P., et al. Effect of increasing doses of linoleic and alpha-linolenic acids on high-fructose and high-fat diet induced metabolic syndrome in rats. Journal of Agricultural and Food Chemistry. 2016;64(4):762–772. doi: 10.1021/acs.jafc.5b04715. [DOI] [PubMed] [Google Scholar]
- Zhou P.-P., Wang M.-Q., Xie F.-J., Deng D.-F., Zhou Q.-C. Effects of dietary carbohydrate to lipid ratios on growth performance, digestive enzyme and hepatic carbohydrate metabolic enzyme activities of large yellow croaker (Larmichthys crocea) Aquaculture. 2016;452:45–51. doi: 10.1016/j.aquaculture.2015.10.010. [DOI] [Google Scholar]
- Zhu S.-J., Gao W.-H., Wen Z.-Y., Chi S.-Y., Shi Y.-H., Hu W., et al. Partial substitution of fish meal by Clostridium autoethanogenum protein in the diets of juvenile largemouth bass (Micropterus salmoides) Aquaculture Reports. 2022;22 doi: 10.1016/j.aqrep.2021.100938. [DOI] [Google Scholar]
- Zuo R., Mai K., Xu W., Turchini G.M., Ai Q. Dietary ALA, but not LNA, increase growth, reduce inflammatory processes, and increase anti-oxidant capacity in the marine finfish Larimichthys crocea. Lipids. 2015;50(2):149–163. doi: 10.1007/s11745-014-3970-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.