Significance
Empathy and social support are key factors influencing pain and are central to the patient–clinician interaction. Yet, our knowledge about the underpinning mechanisms is mostly based on single-subject studies. We recorded simultaneous brain activity in chronic pain patients and clinicians who interacted while patients received evoked pain. Patients received pain in isolation or in presence of a supportive clinician. In half of the dyads patient–clinician pairs, therapeutic alliance was boosted through a prior clinical interaction. Patients’ pain intensity was reduced while interacting with a clinician relative to being alone. Prior clinical interaction increased patients’ brain activation in prefrontal/somatosensory circuitry and increased patient–clinician concordance in brain activity. Our findings suggest a two-brain mechanism underpinning pain empathy and supportive care.
Keywords: Pain, fMRI, hyperscanning, empathy, supportive care
Abstract
Social interactions such as the patient–clinician encounter can influence pain, but the underlying dynamic interbrain processes are unclear. Here, we investigated the dynamic brain processes supporting social modulation of pain by assessing simultaneous brain activity (fMRI hyperscanning) from chronic pain patients and clinicians during video-based live interaction. Patients received painful and nonpainful pressure stimuli either with a supportive clinician present (Dyadic) or in isolation (Solo). In half of the dyads, clinicians performed a clinical consultation and intake with the patient prior to hyperscanning (Clinical Interaction), which increased self-reported therapeutic alliance. For the other half, patient—clinician hyperscanning was completed without prior clinical interaction (No Interaction). Patients reported lower pain intensity in the Dyadic, relative to the Solo, condition. In Clinical Interaction dyads relative to No Interaction, patients evaluated their clinicians as better able to understand their pain, and clinicians were more accurate when estimating patients’ pain levels. In Clinical Interaction dyads, compared to No Interaction, patients showed stronger activation of the dorsolateral and ventrolateral prefrontal cortex (dlPFC and vlPFC) and primary (S1) and secondary (S2) somatosensory areas (Dyadic–Solo contrast), and clinicians showed increased dynamic dlPFC concordance with patients’ S2 activity during pain. Furthermore, the strength of S2-dlPFC concordance was positively correlated with self-reported therapeutic alliance. These findings support that empathy and supportive care can reduce pain intensity and shed light on the brain processes underpinning social modulation of pain in patient–clinician interactions. Our findings further suggest that clinicians’ dlPFC concordance with patients’ somatosensory processing during pain can be boosted by increasing therapeutic alliance.
During negative affective states, such as pain, the presence of a supportive other can be pivotal (1–3). Recent meta-analyses have confirmed that the presence of a friend, romantic partner, or even a supportive stranger can reduce pain intensity (4). The patient–clinician interaction is a context in which pain may be particularly sensitive to psychosocial influences (5–7). Indeed, several studies have found that the social context of the patient–clinician relationship can directly influence pain outcomes and other symptoms (8–11).
While the mechanisms supporting such effects are not well understood, a number of neuroimaging studies have investigated brain processes supporting psychosocial pain modulation (2, 12–14) and vicarious/empathic experience of pain in another (15–19). Social modulation of pain from the first-person perspective (the individual that experiences pain) and vicarious/empathic pain-related processing from the second-person perspective (the individual that interacts with the person in pain) are integrative parts of a dynamic social interaction. Yet, these concepts have largely been studied separately using passive, noninteractive designs and single individuals in isolation (20). It is increasingly acknowledged that studies involving actual interaction, compared to noninteractive social observation, are necessary to identify processes uniquely involved in social interactions (21). Here, we extended this two-person approach to the clinical context to investigate how the patient–clinician relationship can impact the pain experience for chronic pain patients.
We simultaneously recorded brain activity from interacting chronic pain patients (i.e., fibromyalgia) and clinicians under different social contexts using functional MRI (fMRI hyperscanning). In our previous study using this technique, we found that during anticipation of pain treatment, patient–clinician brain concordance in circuitry implicated in social mirroring and theory of mind was increased for dyads that had previously established a clinical relationship (8). In the current study, we investigated how the presence of a clinician impacts patients’ pain and underpinning brain processes. Furthermore, we investigated clinician brain concordance with patients’ somatosensory processing during evoked pain. Chronic pain patients received evoked pressure pain under two conditions: 1) in the presence of a clinician with whom they could interact via a live video-link (Dyadic) and 2) in isolation, without the presence of a clinician, yet otherwise perceptually matched on pain and static visual stimuli (Solo) (Fig. 1). We further compared patient–clinician dyads who had previously established a clinical relationship as part of a clinical consultation and intake prior to the hyperscanning session (Clinical Interaction), relative to dyads who had not previously interacted (No Interaction). We hypothesized that the presence of a clinician would decrease patients’ evoked pain intensity, compared to when patients received pain in isolation. Furthermore, we predicted that improving the therapeutic alliance through the relationship-building clinical intake would improve clinicians’ empathy for, and understanding of, patients’ pain, which in turn would contribute directly to pain relief for the patient. We hypothesized that these psychosocial effects would be underpinned by changes in the patients’ brain processes during pain when socially interacting with a clinician relative to receiving pain in isolation. Finally, we investigated whether a prior clinical interaction modulated brain-to-brain concordance between patients and clinicians.
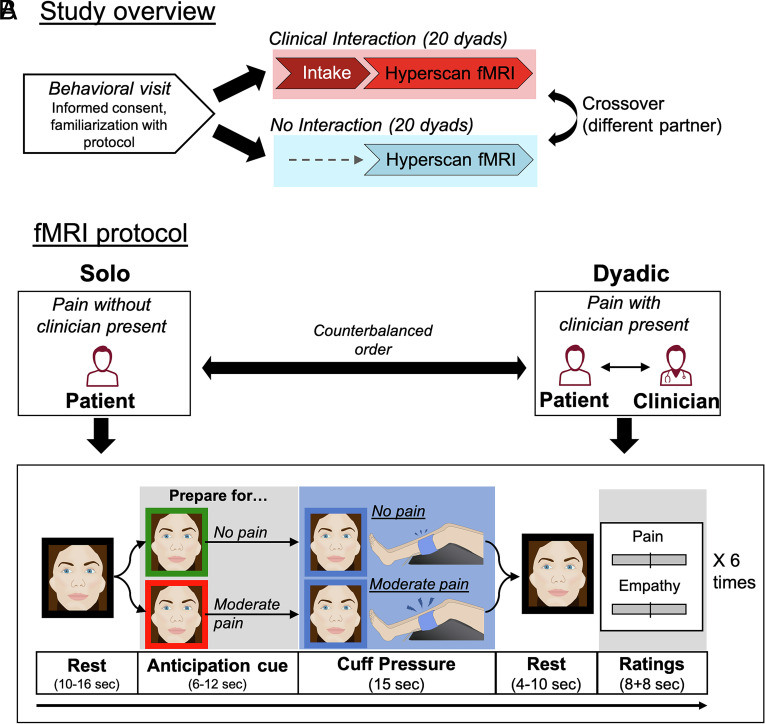
Fig. 1.
Study overview and protocol. (A) After an initial behavioral visit, chronic pain patients and clinicians participated in two separate hyperscanning sessions, in a counterbalanced order, in which they interacted with two different “partners.” For Clinical Interaction dyads, clinicians performed a clinical intake with the patient, on a separate day before the hyperscan, in order to establish a social relationship and a level of therapeutic alliance. In the “No Interaction” control condition, there was no intake, and the patient and clinician were only briefly introduced on the day of the scan. (B) At each of the 2 fMRI visits, patients received a series of nonpainful and moderately painful (individually calibrated) leg pressure stimuli over two separate fMRI scan runs. For each scan run, the patient experienced the same pressure pain paradigm in isolation (Solo), or with their clinician partner present, via real-time, dynamic video connection (Dyadic), in a counterbalanced order. During the Dyadic scan run, both patients and clinicians were scanned simultaneously to assess dynamic concordance in brain activity, while in the Solo run, patients completed a similar experimental procedure without any social interaction, but with a still image of the clinician presented in place of the video stream, to control for sensory-discriminative aspects of the visual stimulation. During each of the fMRI scan runs, participants were shown a visual anticipation cue (red or green frame around the face) followed by Moderately painful (following the red cue) or Nonpainful (following the green cue) cuff pressure applied to the patients left leg, in a pseudorandomized sequence. After each pressure stimulus, patients rated pain intensity (“How painful was the cuff?”) and clinician empathy (“How well did the clinician understand your pain?”), while clinicians rated vicarious pain (“How painful was it for the patient?”) and empathy (“How well did you understand the patient’s pain?”). Ratings were completed using a visual analog scale.
Results
Pain Intensity.
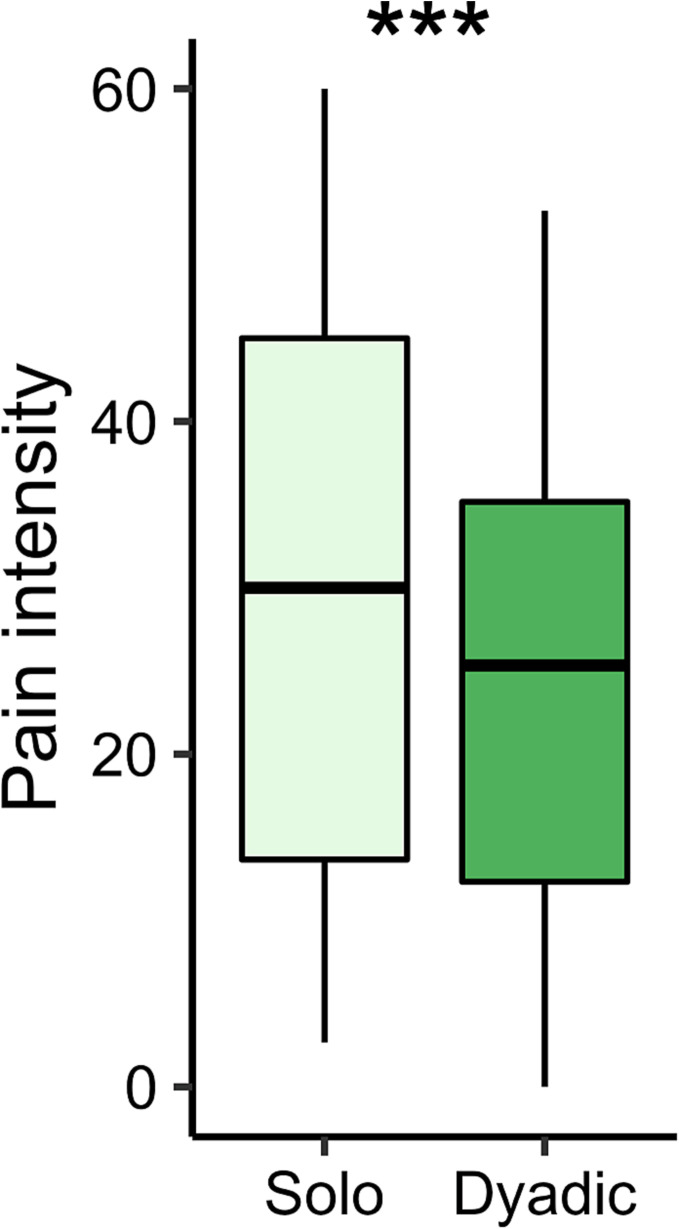
A repeated measures ANOVA indicated that patients’ ratings of pressure pain intensity were reduced during the presence of a clinician (Dyadic, M = 29.43, SD = 17.69), compared to when receiving pain in isolation (Solo, M = 34.27, SD =19.81), F(1,181) = 9.26, P = 0.003, ηp2 = 0.05 (Fig. 2). Contrary to our hypothesis, there was no significant difference in pain intensity between Clinical Interaction dyads (M = 34.07, SD = 19.11), in which the dyad had performed an intake prior to the hyperscan, relative to No Interaction dyads (M = 29.04, SD = 18.33), F(1,181) = 2.44, P = 0.12, ηp2 = 0.01.
Fig. 2.
Patients rated leg cuff pressure as less painful while interacting with their clinician (Dyadic) compared to experiencing pain in isolation (Solo), as indicated by a repeated measures ANOVA. ***P < 0.005
Empathy for Pain.
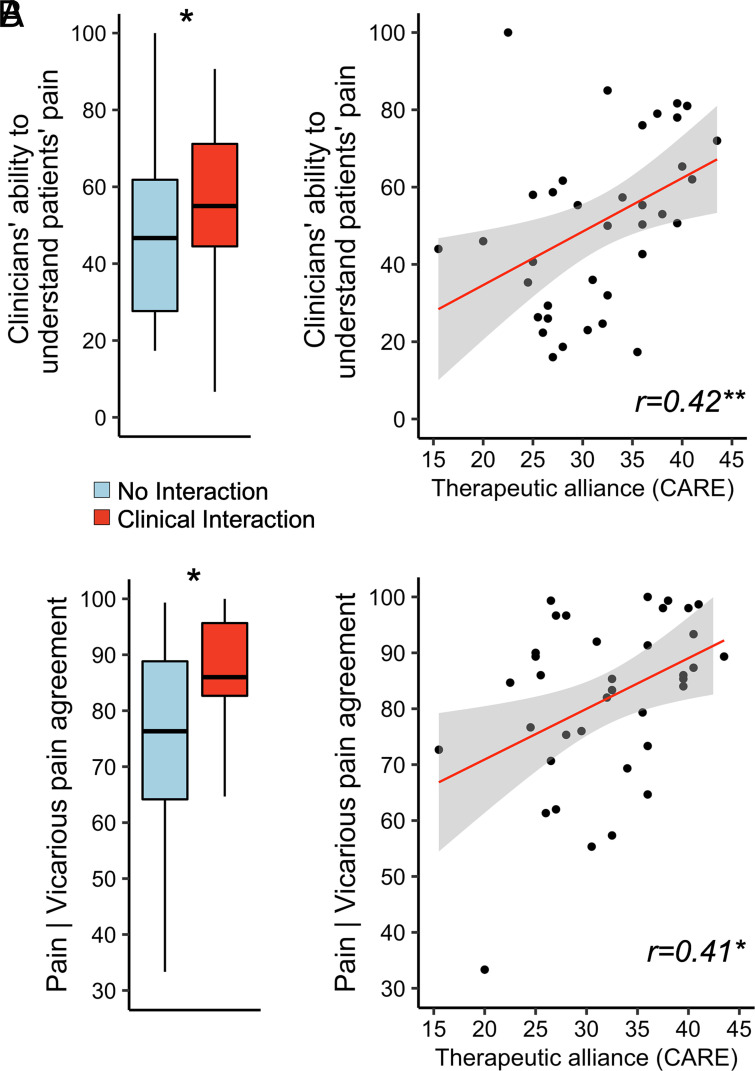
A repeated measures ANOVA indicated that clinicians’ empathy for patients’ pain, as reported by the patient (“How well did the clinician understand your pain?”, Visual Analog Scale, VAS, 0 to 100), was greater for Clinical Interaction (M = 54.33, SD = 26.55) relative to No Interaction (M = 49.29, SD = 26.45), F(1,181) = 5.64, P = 0.019, ηp2 = 0.03 (Fig. 3 A, Left). Furthermore, pain empathy scores correlated positively with therapeutic alliance [CARE questionnaire (22)], such that in dyads with higher therapeutic alliance, patients thought the clinician better understood their pain (r = 0.42, P < 0.01) (Fig. 3 A, Right).
Fig. 3.
Ratings of pain empathy and agreement between patients’ pain and clinicians’ vicarious pain. (A) The clinicians’ ability to understand patients’ pain, as rated by the patient, was significantly higher for Clinical Interaction dyads, compared to No Interaction dyads (Left). Clinicians’ ability to understand patients’ pain was also rated as higher for dyads characterized by higher therapeutic alliance (Right). (B) Correspondingly, patient–clinician agreement in their respective ratings of pain and vicarious pain (100 = perfect agreement, 0 = the least possible agreement, operationalized as the absolute difference between the clinician’s mean vicarious pain vs. the patient’s mean pain intensity, subtracted from 100 (i.e., 100 = perfect agreement, 0 = the least possible agreement) was higher for Clinical Interaction relative to No Interaction dyads (Left). Moreover, Pain | Vicarious pain agreement also correlated positively with therapeutic alliance (Right). CARE = Consultation and Relational Empathy questionnaire. *P < 0.05; **P < 0.01
Agreement between Patients’ Pain and Clinicians’ Vicarious Pain.
In order to investigate clinicians’ accuracy in estimating patients’ level of pain, we calculated the overall agreement between patients’ pain intensity and clinicians’ vicarious pain ratings for each dyad (i.e., the absolute difference between the clinician’s mean vicarious pain and the patient’s mean pain intensity was subtracted from 100, such that 100 = perfect agreement, 0 = the least possible agreement; see Methods for details). A two-way mixed ANOVA showed a main effect of “Clinical context” [F(1, 34) = 5.254, P = 0.028, ηp2 = 0.13], indicating that agreement between patients’ pain and clinicians’ vicarious pain was greater for Clinical Interaction (M = 86.98, SD = 10.09) relative to No Interaction (M = 76.15, SD = 17.47), (Fig. 3 B, Left). There was no significant effect of “Order” [F(1, 34) = 0.003, P = 0.954, ηp2 < 0.01]. Furthermore, Pain | Vicarious pain agreement correlated positively with therapeutic alliance (r = 0.41, P = 0.01) (Fig. 3 B, Right).
Patients’ Brain Response to Leg Pressure Pain.
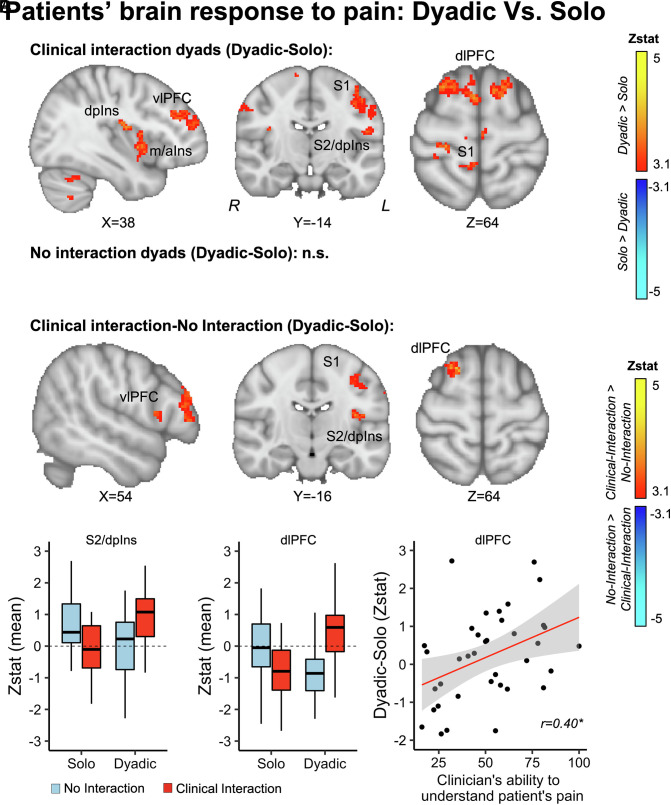
In order to investigate the influence of clinician presence on patients’ brain processing of evoked pain, we compared patients’ BOLD fMRI responses to evoked leg pressure pain while interacting with a clinician (Dyadic), relative to receiving pain in isolation (Solo). In the Dyadic condition relative to Solo, patients in the Clinical Interaction condition showed increased pain-evoked brain responses in left dorsoposterior (dpIns) and mid/anterior (m/aIns) insula (ipsilateral to the leg pressure stimuli), bilateral primary (S1) and secondary (S2) somatosensory areas, and bilateral dorsolateral (dlPFC) and ventrolateral (vlPFC) Prefrontal cortices (Fig. 4A). There were no significant activations in the opposite direction (Solo–Dyadic). Moreover, patients in the No Interaction condition did not show significant differences between Dyadic and Solo (Fig. 4B). Next, we directly compared the Dyadic–Solo contrast for Clinical Interaction relative to No Interaction dyads, to evaluate whether prior clinical relationship significantly influenced these brain processes. For Clinical Interaction relative to No Interaction (Dyadic–Solo contrast), patients showed increased fMRI response in vlPFC, S1, S2/dpIns, and dlPFC (Fig. 4C). Moreover, Z-statistical estimates extracted from each patient’s dlPFC cluster showed a positive correlation with therapeutic alliance (r = 0.40, P = 0.01), such that those with higher increase in dlPFC response during the Dyadic relative to Solo condition reported higher therapeutic alliance with their clinician (Fig. 4 D, Right).
Fig. 4.
Patients’ brain response to pressure pain was modulated by the social interaction between patient and clinician. (A) Patients who were part of Clinical Interaction dyads showed increased activation in bilateral dorsoposterior (dpIns) and left mid-anterior (m/aIns) insula, the bilateral primary (S1) and secondary somatosensory areas (S2), and the right ventrolateral (vlPFC) and bilateral dorsolateral prefrontal cortex (dlPFC) during the Dyadic relative to the Solo condition. There were no significant differences between Dyadic and Solo for No Interaction dyads (B). (C) When comparing Clinical Interaction and No Interaction dyads directly, there was increased BOLD fMRI response in vlPFC, S1, S2/dpIns, and dlPFC for Clinical Interaction, relative to No Interaction, and no significant differences for the opposite contrast. (D) Extracted mean Zstat values from S2/dpIns and dlPFC illustrate the directionality of this effect. Specifically, patients who showed stronger increases in dlPFC activation for Dyadic relative to Solo also reported that their clinician understood their pain better. *P < 0.05.
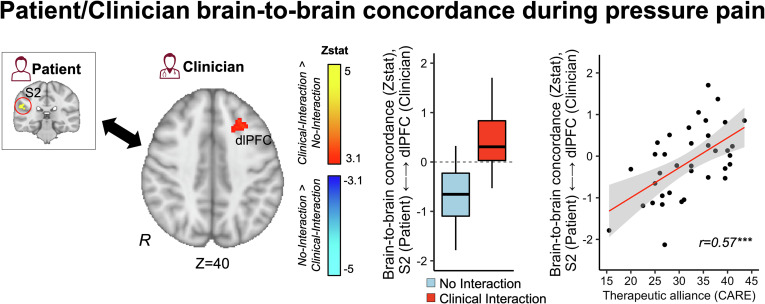
Brain-to-Brain Concordance.
In order to investigate clinicians’ brain activity concordance with patients’ pain processing circuitry, we first extracted patients’ fMRI response from a binarized mask of the contralateral S2. This region of interest was identified as the most prominent cluster in the Moderate pressure pain Vs. Nonpainful pressure contrast calculated from an independent fMRI scan run, without social interaction (Solo) (SI Appendix, Fig. S1). Next, for each hyperscan dyad, we used the patient’s S2 time-series as a regressor of interest in the clinician’s brain first-level general linear model and calculated the interaction term between this regressor and experimental blocks (i.e., anticipation cues, Moderate pain, No pain, rating periods) as an estimate of the clinician’s concordance with the patient’s S2 activity during specific events (see Methods for further details). For Clinical Interaction relative to No Interaction dyads, clinicians showed increased dlPFC concordance with patients’ S2 activity during pain (Fig. 5). Furthermore, the magnitude of S2-dlPFC concordance was positively correlated with therapeutic alliance (r = 0.57, P < 0.001).
Fig. 5.
Patient/clinician concordance in brain activity during pain. In order to assess clinician concordance with patients’ pain-related brain activity during evoked pain, we first extracted the time series of the patient’s S2 activity from a cluster identified from an independent Solo fMRI scan run (Moderate pain–No pain). For each clinician’s fMRI data, we calculated the interaction term between the patients’ S2 time series and a binary function for the leg pressure stimuli. The outcome of this interaction term reflected the clinicians’ voxelwise time-dynamic concordance with the patient’s S2 activity during pain. For Clinical Interaction dyads, compared to No Interaction, clinicians showed increased dlPFC concordance with patients’ S2 activity (Left). The strength of S2-dlPFC concordance was positively correlated with therapeutic alliance (Right). ***P < 0.005.
Discussion
Although the potential of the patient–clinician interaction to influence pain outcomes is widely acknowledged, the brain-behavioral mechanisms driving such effects are not well understood. Our study applied a fMRI hyperscanning approach to investigate the brain-to-brain processes by which social interaction with a clinician can impact chronic pain patients’ nociceptive processing. Overall, patients’ evoked pain ratings were reduced while interacting with a clinician (Dyadic condition) relative to receiving pain in isolation (Solo condition). Moreover, in the Dyadic relative to Solo condition, patients showed increased fMRI response to evoked pressure pain in multiple nociceptive and pain modulatory brain regions, including the dorsoposterior insula (dpIns), primary (S1) and secondary (S2) somatosensory cortices, and ventrolateral (vlPFC) and dorsolateral (dlPFC) prefrontal cortices. This effect was more pronounced for Clinical Interaction dyads, in which therapeutic alliance was increased through prior interaction during a clinical intake compared to No Interaction control dyads. Importantly, our hyperscan fMRI analysis found that clinicians showed increased dlPFC concordance with patients’ brain activity in contralateral S2, the region identified in patients as most responsive to experimental pain in a separate Solo fMRI scan run. Our results suggest a brain-to-brain neural mechanism supporting social modulation of pain inherent to the patient–clinician interaction.
Our finding that patients reported less evoked pain intensity to identical pressure stimuli in the Dyadic relative to the Solo condition is consistent with multiple prior studies, which have found that the presence of another (supportive) individual can provide analgesic influence (2–4, 23, 24). However, this literature is methodologically heterogeneous, with many variables influencing the effect, such as the social relationship with the partner (e.g., clinician, romantic partner, friend, or stranger), the social context (e.g., pain treatment, a painful clinical intervention, or the impact of social interaction on ongoing pain), and the pain modality (experimental or clinical pain) (25–29). Of particular interest in the context of the present study is whether the patient–clinician interaction has a unique or qualitatively different effect on symptoms such as pain, compared to nonclinical supportive relationships. A recent meta-analysis investigated the influence of social support on experimental pain outcomes in nonclinical contexts, e.g., in the presence of a friend, romantic partner, or stranger. This synthesis did not find an overall effect of the presence of another person on pain outcomes such as pain intensity, unpleasantness, and tolerance (4). However, social support did have an overall small-to-moderate effect of decreasing pain-related physiological arousal. In contrast to most other kinds of (supportive) relationships, the patient–clinician relationship is characterized by relatively well-defined roles in which the patient seeks help while the clinician attempts to provide relief or treatment. Thus, the clinician’s behavior and expressions may be particularly potent signals conveying safety or hope to the patient, which may translate to symptom reduction (5, 30, 31), or down-regulated nociceptive processing (2, 8, 14). Indeed, a recent meta-analysis of clinical studies found significant small-to-moderate effects of boosting clinician empathy and facilitating positive patient–clinician communication on a range of patient outcomes (10). Another meta-analysis found that clinical communication training for clinicians had a moderate beneficial effect on patient outcomes (32).
Although patients in Clinical Interaction dyads, relative to No Interaction dyads, reported feeling better understood by the clinician and rated higher therapeutic alliance, we did not find a difference in evoked pain ratings between these two conditions. Importantly, we did not systematically manipulate the clinicians’ behavior during patient interaction in our study. Instead, clinicians were instructed to perform their prescan clinical intake and evaluation in a naturalistic manner, “as closely as possible to a real intake session”. It is possible that active boosting of the relationship with respect to, for example, warmth/empathy (9, 33) or clinician competence (34, 35), may lead to further reduction in pain when contrasting Clinical Interaction relative to No Interaction dyads. Previous studies have reported mixed results as to whether relationship quality itself affects pain relief or other aversive outcomes/negative affect (e.g., stress) (4, 36, 37). A previous study investigating the impact of social support on experimental pain outcomes found that having a supportive partner present, compared to receiving pain in isolation, reduced pain intensity. However, this effect did not differ depending on whether this person was a friend or a stranger (24). Another study investigated the effect of social support on cardiovascular reactivity to stress and found an equally large effect for participants whose supportive partner was a friend vs. a stranger (38). These studies suggest that although having a supportive partner present may be helpful, the specific relationship with this person may have less of an impact on pain outcomes. Interestingly however, a recent study compared evoked pain in the presence of a supportive confederate relative to a neutral nonsupportive confederate. Pain intensity and salivary cortisol were significantly decreased in the supportive condition, suggesting that the mere presence of another individual may not be sufficient (27). Future studies are needed to specify how different aspects of the preexisting relationship affect the therapeutic context, and, in turn, patient outcomes. Importantly, sociocultural concordance or discordance can play a central role in patient–clinician interactions and can impact a wide range of psychological and somatic outcomes (39–43). While we were not able to formally match patients and clinicians in our study due to the limited number of locally available acupuncturists, this is a key issue that should be the target of future hyperscanning studies. In particular, a central hypothesis for future inquiry would be whether differences in brain-to-brain concordance may underpin differences in gender or ethnic/race concordance (43, 44).
When contrasting the Dyadic relative to Solo context, patients showed increased activation of dorsoposterior and mid-anterior insula, vlPFC, dlPFC, and S1/S2. However, this was only found for Clinical Interaction dyads. When directly contrasting Clinical Interaction with No Interaction dyads, dlPFC, vlPFC, S1, and S2/dpIns survived correction for multiple comparisons. A previous study of healthy volunteers found that pain intensity of evoked noxious heat stimuli was reduced when participants listened to empathic relative to unempathic or neutral, statements (45). This effect was accompanied by increased fMRI response in dlPFC, aIns, and anterior mid-cingulate during pain. Another study found that greater “relationship closeness” was associated with stronger activation in the dlPFC and aIns in healthy individuals who observed their romantic partner receiving pain (19). These studies support the relevance of dlPFC, a cortical region implicated by our own study, in social modulation of pain. The dlPFC, along with aIns, the ventromedial PFC, and vlPFC, also constitute a prefrontal component of a network supporting expectancy- or attention-induced pain reduction (i.e., placebo analgesia) (46–49).
Synchronization of behavior and physiological signals is a fundamental feature of social interaction (50–52) and is thought to support social affiliation (53). Here, we found that clinicians in Clinical Interaction dyads, relative to No Interaction dyads, showed stronger dlPFC concordance with patients’ S2, contralaterally to the pressure stimuli, a region that was identified as most responsive to moderate pain in an independent Solo fMRI scan run. The S2 is a key nociceptive processing cortical region that consistently responds to evoked pain stimuli (54). Moreover, transcranial magnetic stimulation of S2 contralaterally to pain stimulation disrupts evaluation of stimulus intensity, suggesting that S2 may be specifically involved in processing sensory features of pain (55). On the other hand, dlPFC is involved in a range of higher-order cognitive and executive functions (56, 57), including social cognition (58, 59), empathy for negative affect (60), and theory of mind (61). Recent meta-analyses have found dlPFC among commonly activated regions associated with empathy for pain conditions, relative to nonpain control conditions (62). Some studies have suggested that dlPFC may support “cognitive” features of theory of mind, such as evaluation of others’ thoughts and intentions, while affective aspects of theory of mind may rely more on ventromedial prefrontal cortices and other regions such as the aIns and temporoparietal junction (TPJ) (63). For example, repetitive TMS stimulation of the dlPFC has been shown to disrupt task outcomes reflective of cognitive but not affective theory of mind (64, 65). Several lesion studies also support this dissociation (66–68). Interestingly, a recent fMRI study found that medical doctors showed increased activation of dlPFC, along with vlPFC and TPJ, while they applied pain treatment to a confederate “patient” who received evoked pain (15). Recent hyperscanning studies have found increased dyadic concordance involving the dlPFC during collaborative/cooperative tasks relative to noncooperative or competitive conditions (69–74). A recent study, using a between-group competitive context, found that within-group synchronization involving the dlPFC supported in-group bonding (75). Hence, our finding that dynamic coupling between clinicians’ dlPFC and patients’ S2 was increased during Clinical Interaction, and was associated with therapeutic alliance, may reflect a mechanism in which higher-order social processing by the clinician dynamically aligns with somatosensory processes supporting patients’ pain experience.
Our prior study found that patient–clinician concordance in brain activity (e.g., aIns, vlPFC, and TPJ) was increased for Clinical Interaction relative to No Interaction dyads (8) when clinicians applied treatment aimed to reduce patients’ evoked pain. However, for that study we used a slightly different analysis approach wherein dynamic brain concordance was calculated at the level of block trials. For the current study, we calculated brain concordance on a TR-to-TR level due to the limited number of block stimuli per condition (SI Appendix, Supplementary Materials and Methods). An advantage of the current TR-to-TR approach is the increased sensitivity to moment-to-moment dynamics in concordance, which may temporally match well with the behavioral dynamics in this social context (76). Nevertheless, exploratory correlations (SI Appendix, Table S1) between the current approach and previously reported block-wise concordance metrics show moderate-to-high coefficients, supporting the assumption that these two approaches do indeed tap into shared underlying social processes.
There are several limitations to our study. First, clinicians and patients developed their relationship during a single naturalistic clinical intake. In clinical practice, alliance and rapport may develop and strengthen over time, across repeated visits. Future studies should investigate how psychosocial effects on pain and the underpinning brain-to-brain dynamics develop over time. Second, since we were unable to preassign participants to meet a balanced ratio of socioculturally concordant and discordant dyads, the sample included a larger number of socioculturally concordant (i.e., same sex/ethnicity for patient and clinician) relative to discordant dyads. Importantly, however, the proportion between concordant vs. discordant dyads was not significantly different between Clinical Interaction and No Interaction groups (SI Appendix, Supplementary Results), suggesting that our results were not strongly influenced by unequal allocation to Clinical Interaction vs. No Interaction subgroups. Third, we were not able to assess brain activity “during” verbal interaction. Future investigations may apply alternative hyperscanning approaches using different techniques such as electroencephalography and near-infrared spectroscopy, which enable simultaneous recording of brain activity during verbal interactions while participants are in the same room. However, these complementary imaging modalities have other limitations such as comparatively reduced spatial resolution and limited assessment of deeper brain regions. Forth, due to the complex logistics of the interactive hyperscan setup and enrollment of real patient–clinician dyads, the final sample size was limited (n = 37 analyzable dyads; n = 20 patients and n = 20 clinicians). This may have led to insufficient statistical power and thus an increased risk of type II error. Finally, future studies should strive to include an equal sampling of men with fibromyalgia, to disentangle possible sex/gender differences.
In conclusion, our findings support prior research suggesting that a supportive clinical relationship and empathy can foster therapeutic alliance and reduce pain intensity. Our study also sheds light on the brain processes supporting social modulation of pain inherent to the patient–clinician interaction. Our findings further suggest that clinicians’ dlPFC dynamic brain concordance with patients’ somatosensory processing during pain can be boosted by increasing therapeutic alliance.
Methods
Subjects.
Our study enrolled 23 female chronic pain patients [age: 39.95 ± 10.93 (Mean ± SD); race/ethnicity: 18 Caucasian, 2 Hispanic, 2 African American, and 1 multiracial] diagnosed with fibromyalgia for at least 1 y and meeting the Wolfe et al. (77) criteria. Fibromyalgia is highly female-predominant—a recent review found a 10:1 ratio of female-specific to male-specific studies (78). Because there are very few brain-imaging studies of men with fibromyalgia, it is unknown whether there might be sex differences in brain processing of nociception and pain for fibromyalgia. However, for chronic pain more broadly, a recent review of neuroimaging studies concluded that there may be sex differences in chronic pain-related alterations in sensorimotor, insula, and anterior cingulate cortices (78). We therefore aimed to reduce the potential variability added by not including a likely small subgroup of male fibromyalgia participants in this study. We also enrolled 22 licensed acupuncturists who had completed at least a 3-y Masters-level program or were currently in the final year of training (age: 44.32 ± 12.81; 15 female; race/ethnicity: 18 Caucasian, 1 Hispanic, 1 African American, 1 Asian, and 1 multiracial). Fibromyalgia patients were recruited through advertisements, patient lists from pain clinics, and through internal registries of patients who had previously taken part in other research studies at our center. Acupuncture clinicians were recruited through advertisements and directed recruitment at the local acupuncture college. The primary reason for enrolling chronic pain patients and trained clinicians was to increase the opportunity for dyads to establish a clinically relevant relationship during the intake/consultation, as a means of maximizing the overall ecological validity of the study. Furthermore, psychological and social factors are central to the psychopathophysiology of fibromyalgia, which made this patient group an appropriate choice to explore patient–clinician empathy. The decision to enroll acupuncturists as clinicians was motivated by two main reasons. First, a separate experiment embedded in our project involved electroacupuncture treatment (8), a treatment modality that enables on/off treatment during scanning and is thus well equipped for block-design fMRI experiments. Thus, enrolling acupuncturists as clinician participants allowed for the clinicians to apply a treatment relevant to their own practice, which again served to increase ecological validity.
Treatment guidelines for fibromyalgia usually recommend a combination of pharmacological treatment, integrative medicine therapies such as acupuncture, and other nonpharmacological approaches such as exercise, manual psychoeducation, and psychological therapy (79–81). A recent Cochrane review concluded that there was low-to-moderate level evidence that acupuncture improves pain and stiffness in fibromyalgia compared to no treatment and standard care (82). In the United States, chronic pain is the most common indication for acupuncture, and acupuncture schools commonly include fibromyalgia as the most common, prototypical nociplastic pain disorder seen by acupuncturists in clinic (83). Thus, fibromyalgia was an appropriate patient group for the acupuncture treatment used in the current study. Patients and clinicians received monetary remuneration for their participation.
Each participant was paired with two different partners with whom they interacted as part of two separate MRI sessions (Clinical Interaction and No Interaction, Fig. 1). These sessions were identical except for whether or not the dyad had previously interacted as part of a clinical intake (see details below). At least 1 MRI visit was completed by 20 patients and 20 clinicians. After 1 MRI visit, 3 patients (2 due to scheduling issues and 1 due to claustrophobia) and 3 clinicians (2 due to scheduling issues and 1 due to scanner discomfort) dropped out. However, all these participants completed both Solo and Dyadic runs on the initial MRI visit. In total, 40 unique dyads were scanned; however, 2 MRI sessions were incomplete due to scanner malfunction, and 1 MRI session was discontinued mid-scan due to patient withdrawal (due to claustrophobia). Thus, the final sample for analysis consisted of 37 dyads (19 Clinical Interaction and 18 No Interaction) with usable single-person and hyperscan MRI data. Our study was approved by the Institutional Review Board at Massachusetts General Hospital, and all participants provided informed consent before participation.
We were not able to estimate power using dyad-based metrics due to the lack of previous data on brain-to-brain concordance with similar context. However, based on previous data from clinicians observing a “patient” confederate receiving evoked heat pain and applying treatment for alleviating this pain (15), we found a mean blood oxygen level–dependent (BOLD) percent change (within-subjects) for a vicarious pain (“no treatment”) and nonpain (“treatment”) condition of 1.25 ± 1.53 (mean ± SD), which, from the clinicians’ perspective, may partially resemble the moderate pain vs. nonpainful pressure conditions in this study. As previously reported (8), an a priori power analysis (paired, two-tailed, = 0.05) indicated that 15 subjects would be required for 85% power to detect this effect size (RStudio, function pwr.t.test, package pwr). Importantly, that study included a different gender composition (10 females and 8 males) than the current study.
Study Protocol.
After an initial behavioral visit in which the experimenter familiarized the participants with the experimental setup and completed a cuff pain calibration procedure (see below), each participant completed 1) a clinical intake visit followed by 2) a Clinical Interaction MRI with the same dyadic partner from the intake, and 3) a No Interaction MRI visit with a separate dyadic partner and no preceding intake. These three sessions were completed on separate days, and the order between Intake followed by Clinical Interaction MRI and the No Interaction MRI was counterbalanced between participants.
Clinicians were instructed to perform the clinical intake “as similarly as possible to your daily practice” and were not given any time restrictions, in order to maximize ecological validity (mean ± SD duration of intakes: 37:40 ± 12:30 min:s, range: 21:32 to 54:40). The purpose of the intake was to enable the patient–clinician dyads to establish a social relationship and a level of therapeutic alliance prior to the Clinical Interaction MRI session. The No Interaction MRI session, which was not preceded by an intake, was included as a control condition. Each participant was paired with a different partner for these two MRI sessions to avoid carryover effects due to the relationship.
The two MRI sessions followed the same procedure. After the patient had been positioned in the MRI scanner, the clinician facilitated acupuncture needling (see ref. 8 for full details). In brief, the patient received two MR-compatible titanium needles (DongBang, Seongnam, Korea) above the knee proximal to the cuff, with MR-compatible electrodes attached to each needle. These needles stayed in place throughout the entire MRI session and were used for a separate treatment run in which the clinician provided electroacupuncture treatment to alleviate the patient’s cuff pain (see ref. 8). Although there was no treatment element in the current study, the patients’ awareness that they would subsequently receive pain treatment by the same clinician may have improved the participants’ appraisal of the current experimental context as a meaningful clinical interaction. The clinician was then positioned in another MRI scanner in the same building. In both scanners we employed the same customized coil configuration, using the bottom of a 64-channel head coil and a 4-channel flex coil wrapped over the subjects’ forehead to cover the frontal lobes of the brain. This was done in order to ensure unimpeded facial coverage for video transfer. MRI-compatible video cameras enabled the participants to communicate nonverbally (e.g., eye movement and facial expressions) during the experimental hyperscanning runs. Before the scan, the patient and the clinician were instructed that they could freely communicate their feelings to the other person nonverbally (e.g., facial expressions and eye movement) as long as they kept their head as still as possible. Prior to functional MRI (fMRI) scanning for the Clinical Interaction session, the clinician was given the option to “check in” with the patient via the between-scanner audio/video connection, in order to reinforce the clinical relationship. As previously reported (8), patients and clinicians filled out the Hyperscan Relationship Scale, addressing their subjective experience of different aspects of the in-scanner social interaction after each fMRI hyperscanning session. For one of these items, “I felt I could communicate with the patient/acupuncturist” (VAS, 0 to 10; anchors, “Completely disagree” and “Completely agree”), Clinical Interaction dyads rated their ability to communicate with the partner higher (Patients: 7.79 ± 2.64; Clinicians: 5.36 ± 2.32) compared to No Interaction dyads (Patients: 6.56 ± 3.12; Clinicians: 3.75 ± 2.54), and this item correlated positively with Therapeutic alliance ratings from the clinical intake (Patients: r = 0.18; Clinicians: r = 0.47), suggesting that perceived ability to communicate was associated with the clinical relationship.
Evoked Pain Stimuli.
We applied deep-tissue leg pain using the Hokanson Rapid Cuff Inflator (D. E. Hokanson, Inc.). Compared to cutaneous quantitative sensory testing (QST) techniques (e.g., contact heat), deep sustained pain may better mimic clinical pain (84, 85), thus providing a more clinically relevant measure. In our experience, applying these techniques in chronic pain patients, even subjects with severe fibromyalgia are generally able to tolerate leg cuff pain without any lasting discomfort (86, 87). Each patient went through a pain calibration procedure at the initial behavioral visit to determine an individual level of moderate pain (~40 out of 100) to be used for experimental testing. Throughout all the experimental runs, this level was confirmed to evoke the target pain level of “Moderate pain” (mean ± SD = 157.92 ± 95.59 mmHg), while a mild pressure (30 mmHg) was used as a “Nonpainful” control stimulus. The cuff was attached to the patient’s left lower leg prior to scanning and was inflated for six 15-s duration trials (3× Moderate pain, 3× No pain) during the experimental runs (Solo, Dyadic).
Self-Report Assessments.
Therapeutic alliance.
Patients and clinicians completed a “Consultation and Relational Empathy (CARE)” questionnaire (22) at the end of each MRI session. The total scores for patient-rated and clinician-rated (88, 89) CARE were calculated and averaged for each dyad and used as an estimate of dyad-wise therapeutic alliance.
Ratings of pain, vicarious pain, and empathy for pain.
At the end of each trial, participants used a MRI-compatible button box to provide ratings (8 s each) using a visual analog scale (VAS, quantified as 0 to 100). During Solo runs, patients rated pain intensity (“How painful was the cuff?” Anchors: “No pain” and “Most pain imaginable”). During Dyadic runs, patients rated pain intensity (“How painful was the cuff?” Anchors: “No pain” and “Most pain imaginable”) and empathy for pain (“How well did the clinician understand your pain?” Anchors: “Not at all” and “Extremely well”). Clinicians rated vicarious pain (“How painful was it for the patient?”) and empathy for pain (“How well did you understand the patient’s pain?” Anchors: “Not at all” and “Extremely well”).
MRI Acquisition and Preprocessing.
MRI acquisition.
BOLD fMRI data were simultaneously collected from each participant (Patient scanner: Siemens 3T Skyra; Clinician scanner: Siemens 3T Prisma) using a whole-brain, simultaneous multislice, T2*-weighted gradient echo-planar imaging pulse sequence (repetition time = 1,250 ms, echo time = 33 ms, flip angle = 65˚, voxel size = 2 mm isotropic, number of slices = 75, multiband acceleration factor = 5, 624 volumes split into two consecutive scan runs). We decided to keep a designated “patient scanner” and “clinician scanner” rather than randomizing scanner assignment between dyads. This allowed for protocol consistency within patient and clinician groups and facilitated the setup of our hyperscanning infrastructure. In order to maximize patient comfort during scanning, we used the Siemens 3T Skyra for the fibromyalgia patient group, since it has a slightly wider bore compared to the Siemens 3T Prisma.
A T1-weighted high-resolution structural volume (multiecho MPRAGE) was collected to facilitate anatomical localization and spatial registration of individual BOLD fMRI volumes to standard space (Montreal Neurological Institute, MNI152) (repetition time = 2,530 ms, echo time = 1.69 ms, flip angle = 7˚, voxel size = 1 mm isotropic).
MRI preprocessing.
Individual fMRI datasets were preprocessed using tools from FMRIB’s Software Library (FSL, v6.0.0; www.fmrib.ox.ac.uk/fsl) and included the following steps: slice-timing correction, motion correction (MCFLIRT) (90), correction of spatial inhomogeneity (TOPUP) (91, 92), nonbrain tissue removal (BET) (93), spatial smoothing (full width at half maximum = 4 mm), temporal high-pass filtering (f = 0.011 Hz as computed by FSL’s cutoffcalc), and grand-mean intensity normalization by a single multiplicative factor. For each subject, both runs were realigned (6 degrees of freedom) to a common reference space (7th volume of the first run) before the first-level GLM analyses. The transformation matrix for registration between functional and high-resolution anatomical volumes was calculated using Boundary Based Registration [bbregister, Freesurfer, v6.0.0 (94)]. Due to excessive head motion, two patients and one clinician had one of their Dyadic runs excluded from fMRI analysis, and one patient had one of their Solo runs excluded, based on the following exclusion criteria: 1) >2˚ frame-by-frame head rotation in any direction and 2) >2 mm frame-by-frame displacement. For registration from structural to standard space (MNI152), we used FSL’s Linear registration tool (FLIRT, 12 degrees of freedom) (90, 95), followed by FSL’s nonlinear registration tool (FNIRT) (96). All single-subject analyses were performed in functional space and then registered to MNI152 standard space before dyadic and group analyses.
Materials.
In-scanner cameras.
Each MRI scanner was equipped with an MRI-compatible camera (Model 12M, MRC Systems GmbH, Heidelberg, Germany) attached to the table-mounted mirror in order to enable online visual communication. Prior to scanning, cameras were manually adjusted to capture the full face of each participant. The visual stream was projected onto a screen behind the MRI scanner bore, which the participants viewed through the table-mounted mirror. The two-way video stream (20 Hz) was transmitted over a local network (the cross-scanner delay was measured by our custom in-house software to be consistently <40 ms).
Microphones.
Although verbal communication was disabled during scanning to avoid speech-related motion artifacts in the fMRI signal, participants were able to communicate verbally between the Solo and Dyadic MRI scan runs. Speech was recorded using MRI-compatible optical microphones (Fibersound FOM1-MR, Micro Optics Technologies Inc.).
Software for stimulus presentation and signal synchronization.
We applied in-house software (C++) for synchronization of fMRI and video signal acquisition between MRI scanners, transferring video and audio, and tracking the between-scanner network delay. A laptop in each MRI scanner control room initiated fMRI acquisition using a remote trigger. These laptops also locally controlled the video stream, experimental visual stimuli, onset and offset of the leg pressure, and recording in-scanner ratings and videos, thus using a common clock for synchronization. The two laptops were connected through a local area network for bidirectional communication. At the initiation of each fMRI pulse sequence, a signal from the master computer (patient MRI control room) was sent to the slave computer (clinician MRI control room). The current network delay (calculated as the mean of 10 network pings) was estimated, and the timer clock and the fMRI pulse sequences were then initiated and locally adjusted for this network lag, thus ensuring synchronized acquisition timing of the two fMRI time series, video streams, and experimental protocols. The video streams were uncompressed to minimize a computational delay, and the brightness of display was adjusted to the same level.
Statistical Analysis.
All nonimaging analyses were performed in R (RStudio 1.4.1106). The threshold for statistical significance was set at .
Self-report data.
To investigate whether patients’ pain intensity was affected by the presence of the clinician or whether the dyad had interacted during a prior clinical intake, we performed a repeated measures ANOVA with factors “Run” (Solo, Dyadic) and “Clinical context” (Clinical Interaction, No Interaction) and “Order” as a between-subjects factor (Clinical Interaction first, No Interaction first).
To investigate the clinicians’ ability to understand patients’ pain, as rated by the patient, we performed a repeated measures ANOVA with factors “Clinical context” (Clinical Interaction, No Interaction) and “Stimulus” (Moderate pain, No pain) and “Order” as a between-subjects factor (Clinical Interaction first, No Interaction first).
Agreement between patients’ pain and clinicians’ vicarious pain.
In order to assess clinician accuracy in estimating patients’ pain, we calculated the agreement between the patients’ pain ratings and clinicians’ vicarious pain ratings. For each dyad, we first calculated the absolute difference between the clinician’s mean rating of vicarious pain and the patient’s mean rating of pain intensity. These absolute difference scores were then transformed by subtracting each score from 100 (the largest possible difference), such that a score of 0 would indicate the largest possible difference, while 100 would indicate perfect agreement between the clinician’s vicarious pain rating and the patient’s pain intensity rating. Thus, one Agreement score for each dyad was used for statistical analysis, consistent with previous studies of empathic accuracy for pain (97, 98). We performed a two-way ANOVA with the within-subjects factor “Clinical context” (Clinical Interaction, No Interaction) and the between-subjects factor “Order” (Clinical Interaction first, No Interaction first) to investigate whether agreement differed between Clinical Interaction dyads and No Interaction dyads.
fMRI data.
For all whole-brain group analyses of fMRI data, significance testing was done using FSL FLAME 1+2 with cluster correction for multiple comparisons (z = 3.1, α = 0.05) (99).
Main contrasts.
We first performed first-level GLM analyses for each individual run (Solo, Dyad) for each subject using FILM with local autocorrelation correction (100). We modeled periods corresponding to cuff stimulation (Moderate pain, No pain) as regressors of interest. Rating periods and six motion parameter time series were modeled as regressors of no interest in the same design matrix. The contrast parameter estimates for the contrast Moderate pain vs. No pain were then passed up to a second-level fixed-effects GLM contrasting Dyadic vs. Solo runs from the same MRI session. These individual whole-brain maps (Dyadic–Solo) were passed up to group analyses for 1) Clinical Interaction dyads, 2) No Interaction dyads, and 3) Clinical Interaction vs. No Interaction. The resulting whole-brain Z-statistical maps showed pain-related BOLD differences while patients were interacting with a clinician (Dyadic), relative to receiving pain in isolation (Solo), in Clinical Interaction dyads, No Interaction dyads, and the difference between these two.
Brain-to-brain concordance.
To investigate clinicians’ concordance with patients’ pain-related brain processing, we first performed a group-level contrast of BOLD responses during Moderate pain vs. No pain for all patients’ Solo runs, in order to identify brain circuitry implicated in pressure pain processing (without being influenced by social interaction). Using a functionally derived mask from an independent localizer task for the same individuals may yield higher spatial sensitivity than an atlas-based mask derived from anatomical landmarks (101–103). Patients varied considerably in their individually calibrated cuff stimulus intensity for Moderate pain (range: 50 to 380 mmHg), which may affect the contrast with nonpainful cuff (always 30 mmHg). In order to maximize the sensitivity to pain-related processing in this analysis, we therefore excluded patients who had a calibrated cuff intensity for Moderate pain of lower than 70 mmHg (n = 4). The resulting group map (n = 16 patients, SI Appendix, Fig. S1) included a cluster in the right secondary somatosensory area (S2), contralateral to cuff pain stimulation, which we used as an independently defined binarized region of interest (ROI) for the concordance analyses.
Specifically, we extracted the mean time series from a binarized mask of this cluster, from each patients’ preprocessed BOLD data registered to MNI152 space (Dyadic run, full sample of 37 dyads, including 20 patients). Next, for each dyad, the patient’s S2 time series was used as a regressor in the clinician’s first-level GLM. Periods corresponding to vicarious pain (observation of the patient receiving Moderate pain) were included as a binary regressor, and we computed the interaction term between the patient’s S2 regressor (de-meaned) and the vicarious pain regressor (centered, such that pain periods corresponded to +1 and nonpain periods corresponded to −1). Other events corresponding to vicarious nonpain (observation of the patient receiving nonpainful leg pressure), anticipation cues, and rating periods were included as regressors of no interest, along with motion parameters. The contrast parameter estimates for the S2Patient*Vicarious Pain interaction term thus yielded a whole-brain map from each dyad showing the clinician’s voxelwise concordance with the patient’s S2 BOLD fluctuations, specifically during evoked pain. This approach is similar to a generalized psychophysiological interaction analysis (104, 105), except that the physiological regressor was derived from the interacting partner’s brain rather than a different brain region in the same individual.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This study was supported by the NIH, the National Center for Complementary and Integrative Health (R61/R33-AT009306), Research Council of Norway/Marie Sklodowska-Curie Actions (FRICON/COFUND-240553/F20), Neuroimaging Pilot Funding Initiative at the Martinos Center for Biomedical imaging, Massachusetts General Hospital (MGH) (R90DA023427), South-Eastern Norway Regional Health Authority (2020040 and 2022086), Korea Institute of Oriental Medicine (KSN2021240, KSN2211010), Foundation for the Science of the Therapeutic Encounter, National Center for Research Resources (P41RR14075; CRC 1 UL1 RR025758). The infrastructure for this study was supported by the Harvard Clinical and Translational Science Center, Martinos Computing facilities, and NIH (grant nos. S10RR023401, S10RR019307, S10RR019254, and S10RR023043). We would like to thank Marco Loggia and Karin B. Jensen for input on research design and Katie Walker for assisting acupuncture administration.
Author contributions
D.-M.E., K.I., C.J., J.L., J.G., I.M., R.S., R.R.E., J.M.K., I.K., T.J.K., and V.N. designed research; D.-M.E., K.I., C.J., J.L., J.G., I.M., R.S., and V.N. performed research; D.-M.E. and C.J. contributed new reagents/analytic tools; D.-M.E. analyzed data; and D.-M.E., K.I., J.L., A.G., A.A., R.R.E., J.M.K., I.K., T.J.K., and V.N. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. S.A.N. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
Anonymized csv, txt data have been deposited in OSF (https://osf.io/5khjn/) (106).
Supporting Information
References
- 1.Kulik J. A., Mahler H. I., Social support and recovery from surgery. Health Psychol. 8, 221–238 (1989). [DOI] [PubMed] [Google Scholar]
- 2.Montoya P., Larbig W., Braun C., Preissl H., Birbaumer N., Influence of social support and emotional context on pain processing and magnetic brain responses in fibromyalgia. Arthritis Rheum. 50, 4035–4044 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Zaza C., Baine N., Cancer pain and psychosocial factors: A critical review of the literature. J. Pain Symptom Manage. 24, 526–542 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Che X., Cash R., Chung S., Fitzgerald P. B., Fitzgibbon B. M., Investigating the influence of social support on experimental pain and related physiological arousal: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 92, 437–452 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Kaptchuk T. J., Hemond C. C., Miller F. G., Placebos in chronic pain: Evidence, theory, ethics, and use in clinical practice. BMJ 370, m1668 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Steinkopf L., Disgust, empathy, and care of the sick: An evolutionary perspective. Evol. Psychol. Sci. 3, 149–158 (2017). [Google Scholar]
- 7.Finset A., “How communication between clinicians and patients may impact pain perception” in Placebo and Pain (Academic Press, 2013), pp. 243–256. [Google Scholar]
- 8.Ellingsen D.-M., et al. , Dynamic brain-to-brain concordance and behavioral mirroring as a mechanism of the patient-clinician interaction. Sci. Adv. 6, eabc1304 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaptchuk T. J., et al. , Components of placebo effect: Randomised controlled trial in patients with irritable bowel syndrome. Br. Med. J. 336, 999–1003 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howick J., et al. , Effects of empathic and positive communication in healthcare consultations: A systematic review and meta-analysis. J. R. Soc. Med. 111, 240–252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuentes J., et al. , Enhanced therapeutic alliance modulates pain intensity and muscle pain sensitivity in patients with chronic low back pain: An experimental controlled study. Phys. Ther. 94, 477–489 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Ellingsen D. M., et al. , Placebo improves pleasure and pain through opposite modulation of sensory processing. Proc. Natl. Acad. Sci. U.S.A. 110, 17993–17998 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarinopoulos I., et al. , Patient-centered interviewing is associated with decreased responses to painful stimuli: An initial fMRI study. Patient Educ. Couns. 90, 220–225 (2013). [DOI] [PubMed] [Google Scholar]
- 14.López-Solà M., Geuter S., Koban L., Coan J. A., Wager T. D., Brain mechanisms of social touch-induced analgesia in females. Pain 160, 2072–2085 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Jensen K. B., et al. , Sharing pain and relief: Neural correlates of physicians during treatment of patients. Mol. Psychiatry 19, 392–398 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallon N., Roberts C., Stancak A., Shared and distinct functional networks for empathy and pain processing: A systematic review and meta-analysis of fMRI studies. Soc. Cogn. Affect. Neurosci. 15, 709–723 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison I., Peelen M. V., Downing P. E., The sight of others’ pain modulates motor processing in human cingulate cortex. Cereb. Cortex 17, 2214–2222 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Tinnermann A., Büchel C., Haaker J., Observation of others’ painful heat stimulation involves responses in the spinal cord. Sci. Adv. 7, eabe8444 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Solà M., Koban L., Krishnan A., Wager T. D., When pain really matters: A vicarious-pain brain marker tracks empathy for pain in the romantic partner. Neuropsychologia 145, 106427 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Schilbach L., et al. , Toward a second-person neuroscience. Behav. Brain Sci. 36, 393–414 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Redcay E., Rice K., Saxe R., Interaction versus observation: A finer look at this distinction and its importance to autism. Behav. Brain Sci. 36, 435–435 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Mercer S. W., Maxwell M., Heaney D., Watt G. C., The consultation and relational empathy (CARE) measure: Development and preliminary validation and reliability of an empathy-based consultation process measure. Fam. Pract. 21, 699–705 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Krahé C., et al. , Attachment style moderates partner presence effects on pain: A laser-evoked potentials study. Soc. Cogn. Affect. Neurosci. 10, 1030–1037 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown J. L., Sheffield D., Leary M. R., Robinson M. E., Social support and experimental pain. Psychosom. Med. 65, 276–283 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Kirschbaum C., Klauer T., Filipp S.-H., Hellhammer D. H., Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom. Med. 57, 23–31 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Duschek S., Nassauer L., Montoro C. I., Bair A., Montoya P., Dispositional empathy is associated with experimental pain reduction during provision of social support by romantic partners. Scand. J. Pain 20, 205–209 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Roberts M. H., Klatzkin R. R., Mechlin B., Social support attenuates physiological stress responses and experimental pain sensitivity to cold pressor pain. Ann. Behav. Med. 49, 557–569 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Jackson T., Iezzi T., Chen H., Ebnet S., Eglitis K., Gender, interpersonal transactions, and the perception of pain: An experimental analysis. J. Pain 6, 228–236 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Block P., Heathcote L. C., Burnett Heyes S., Social interaction and pain: An arctic expedition. Soc. Sci. Med. 196, 47–55 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Steinkopf L., The signaling theory of symptoms: An evolutionary explanation of the placebo effect. Evol. Psychol. 13, 1–12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellingsen D.-M., Kringelbach M. L., Leknes S., Kringelbach M. L., Leknes S., “A neuroscience perspective on pleasure and pain” in Philosophy of Pain (Routledge, London, 2018), pp. 60–77. [Google Scholar]
- 32.Kelley J. M., Kraft-Todd G., Schapira L., Kossowsky J., Riess H., The influence of the patient-clinician relationship on healthcare outcomes: A systematic review and meta-analysis of randomized controlled trials. PLoS One 9, e94207 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelley J. M., et al. , Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosom. Med. 71, 789–797 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howe L. C., Leibowitz K. A., Crum A. J., When your doctor “gets it” and “gets you”: The critical role of competence and warmth in the patient-provider interaction. Front. Psychiatry 10, 475 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashton-James C. E., Tybur J. M., Grießer V., Costa D., Stereotypes about surgeon warmth and competence: The role of surgeon gender. PLoS One 14, e0211890 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coan J. A., et al. , Relationship status and perceived support in the social regulation of neural responses to threat. Soc. Cogn. Affect. Neurosci. 12, 1574–1583 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coan J. A., Schaefer H. S., Davidson R. J., Lending a hand: Social regulation of the neural response to threat. Psychol. Sci. 17, 1032–9 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Fontana A. M., Diegnan T., Villeneuve A., Lepore S. J., Nonevaluative social support reduces cardiovascular reactivity in young women during acutely stressful performance situations. J. Behav. Med. 22, 75–91 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Greenwood B. N., Carnahan S., Huang L., Patient–physician gender concordance and increased mortality among female heart attack patients. Proc. Natl. Acad. Sci. U.S.A. 115, 8569–8574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elliott A. M., Alexander S. C., Mescher C. A., Mohan D., Barnato A. E., Differences in physicians’ verbal and nonverbal communication with black and white patients at the end of life. J. Pain Symptom Manage. 51, 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall J. A., Irish J. T., Roter D. L., Ehrlich C. M., Miller L. H., Gender in medical encounters: An analysis of physician and patient communication in a primary care setting. Health Psychol. 13, 384–392 (1994). [DOI] [PubMed] [Google Scholar]
- 42.Gross R., et al. , The association of gender concordance and primary care physicians’ perceptions of their patients. Women Health 48, 123–144 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Losin E. A. R., Anderson S. R., Wager T. D., Feelings of clinician-patient similarity and trust influence pain: Evidence from simulated clinical interactions. J. Pain 18, 787–799 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldstein P., Losin E. A. R., Anderson S. R., Schelkun V. R., Wager T. D., Clinician-patient movement synchrony mediates social group effects on interpersonal trust and perceived pain. J. Pain 21, 1160–1174 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fauchon C., et al. , Brain activity sustaining the modulation of pain by empathetic comments. Sci. Rep. 9, 8398 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krummenacher P., Candia V., Folkers G., Schedlowski M., Schonbachler G., Prefrontal cortex modulates placebo analgesia. Pain 148, 368–374 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Schenk L. A., Sprenger C., Onat S., Colloca L., Büchel C., Suppression of striatal prediction errors by the prefrontal cortex in placebo hypoalgesia. J. Neurosci. 37, 9715–9723 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vachon-Presseau E., et al. , Brain and psychological determinants of placebo pill response in chronic pain patients. Nat. Commun. 9, 3397 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wager T. D., Atlas L. Y., The neuroscience of placebo effects: Connecting context, learning and health. Nat. Rev. Neurosci. 16, 403–418 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasson U., Frith C. D., Mirroring and beyond: Coupled dynamics as a generalized framework for modelling social interactions. Phil. Trans. R. Soc. B 371, 20150366 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koban L., Ramamoorthy A., Konvalinka I., Why do we fall into sync with others? Interpersonal synchronization and the brain’s optimization principle Soc. Neurosci. 14, 1–9 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Ellingsen D.-M., et al. , Patient–clinician brain concordance underlies causal dynamics in nonverbal communication and negative affective expressivity. Transl. Psychiatry 12, 1–9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Redcay E., Schilbach L., Using second-person neuroscience to elucidate the mechanisms of social interaction. Nat. Rev. Neurosci. 20, 495–505 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wager T. D., et al. , An fMRI-Based neurologic signature of physical pain. N. Engl. J. Med. 368, 1388–1397 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lockwood P. L., Iannetti G. D., Haggard P., Transcranial magnetic stimulation over human secondary somatosensory cortex disrupts perception of pain intensity. Cortex 49, 2201–2209 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller E. K., Cohen J. D., An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Mansouri F. A., Egner T., Buckley M. J., Monitoring demands for executive control: Shared functions between human and nonhuman primates. Trends Neurosci. 40, 15–27 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Sellaro R., Nitsche M. A., Colzato L. S., The stimulated social brain: Effects of transcranial direct current stimulation on social cognition: tDCS and social cognition. Ann. N.Y. Acad. Sci. 1369, 218–239 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Weissman D. H., Perkins A. S., Woldorff M. G., Cognitive control in social situations: A role for the dorsolateral prefrontal cortex. NeuroImage 40, 955–962 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stietz J., Jauk E., Krach S., Kanske P., Dissociating empathy from perspective-taking: Evidence from intra- and inter-individual differences research. Front. Psychiatry 10, 126 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wade M., et al. , On the relation between theory of mind and executive functioning: A developmental cognitive neuroscience perspective. Psychon. Bull. Rev. 25, 2119–2140 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Lamm C., Decety J., Singer T., Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage 54, 2492–2502 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Eslinger P. J., Neurological and Neuropsychological Bases of Empathy. Eur. Neurol. 39, 193–199 (1998). [DOI] [PubMed] [Google Scholar]
- 64.Kalbe E., et al. , Dissociating cognitive from affective theory of mind: A TMS study. Cortex 46, 769–780 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Costa A., Torriero S., Oliveri M., Caltagirone C., Prefrontal and temporo-parietal involvement in taking others’ perspective: TMS evidence. Behav. Neurol. 19, 71–74 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shamay-Tsoory S. G., Aharon-Peretz J., Dissociable prefrontal networks for cognitive and affective theory of mind: A lesion study. Neuropsychologia 45, 3054–3067 (2007). [DOI] [PubMed] [Google Scholar]
- 67.Shamay-Tsoory S. G., Tomer R., Berger B. D., Goldsher D., Aharon-Peretz J., Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cogn. Behav. Neurol. 18, 55–67 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Eslinger P. J., Satish U., Grattan L. M., Alterations in cognitive and affectively-based empathy after cerebral damage. J. Int. Neuropsychol. Soc. 2, 15 (1996). [Google Scholar]
- 69.Fishburn F. A., et al. , Putting our heads together: Interpersonal neural synchronization as a biological mechanism for shared intentionality. Soc. Cogn. Affect. Neurosci. 13, 841–849 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu K., Hao N., When do we fall in neural synchrony with others? Soc. Cogn. Affect. Neurosci. 14, 253–261 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu K., Qiao X., Hao N., Praising or keeping silent on partner’s ideas: Leading brainstorming in particular ways. Neuropsychologia 124, 19–30 (2019). [DOI] [PubMed] [Google Scholar]
- 72.Czeszumski A., et al. , Cooperative behavior evokes interbrain synchrony in the prefrontal and temporoparietal cortex: A systematic review and meta-analysis of fNIRS hyperscanning studies. eNeuro 9, ENEURO.0268-21.2022 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cui X., Bryant D. M., Reiss A. L., NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. NeuroImage 59, 2430–2437 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu K., Xue H., Nozawa T., Hao N., Cooperation makes a group be more creative. Cereb. Cortex 29, 3457–3470 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Yang J., Zhang H., Ni J., De Dreu C. K. W., Ma Y., Within-group synchronization in the prefrontal cortex associates with intergroup conflict. Nat. Neurosci. 23, 754–760 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Jessen S., Kotz S. A., The temporal dynamics of processing emotions from vocal, facial, and bodily expressions. NeuroImage 58, 665–674 (2011). [DOI] [PubMed] [Google Scholar]
- 77.Wolfe F., Häuser W., Fibromyalgia diagnosis and diagnostic criteria. Ann. Med. 43, 495–502 (2011). [DOI] [PubMed] [Google Scholar]
- 78.Gupta A., et al. , Sex-based differences in brain alterations across chronic pain conditions: Sex, Chronic Pain, and the Brain. J. Neurosci. Res. 95, 604–616 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clauw D. J., Fibromyalgia: A clinical review. JAMA 311, 1547–1555 (2014). [DOI] [PubMed] [Google Scholar]
- 80.NHS, Treatment Fibromyalgia (2022). https://www.nhs.uk/conditions/fibromyalgia/treatment/. Accessed 20 April 2023.
- 81.U.S. Department of Veterans Affairs, VHA Pain Management (2023). https://www.va.gov/PAINMANAGEMENT/Providers/index.asp. Accessed 20 April 2023.
- 82.Deare J. C., Acupuncture for treating fibromyalgia. Cochrane Database Syst. Rev. (2013) 10.1002/14651858.CD007070.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang H., et al. , The most commonly treated acupuncture indications in the United States: A cross-sectional study. Am. J. Chin. Med., 46, 1–33 (2018). [DOI] [PubMed] [Google Scholar]
- 84.Rainville P., Feine J. S., Bushnell M. C., Duncan G. H., A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosens. Mot. Res. 9, 265–277 (1992). [DOI] [PubMed] [Google Scholar]
- 85.Curatolo M., Arendt-Nielsen L., Petersen-Felix S., Central hypersensitivity in chronic pain: Mechanisms and clinical implications. Phys. Med. Rehabil. Clin. N. Am. 17, 287–302 (2006). [DOI] [PubMed] [Google Scholar]
- 86.Loggia M. L., et al. , Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheum. 66, 203–212 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim J., et al. , The somatosensory link in fibromyalgia: Functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheum. 67, 1395–1405 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Phillips M., Lorie A., Kelley J., Gray S., Riess H., Long-term effects of empathy training in surgery residents: A one year follow-up study. Eur. J. Pers. Cent. Healthc. 1, 326–332 (2013). [Google Scholar]
- 89.Riess H., Kelley J. M., Bailey R., Konowitz P. M., Gray S. T., Improving empathy and relationalskills in otolaryngology residents: A pilot study. Otolaryngol. Head Neck Surg. 144, 120–122 (2011). [DOI] [PubMed] [Google Scholar]
- 90.Jenkinson M., Bannister P., Brady M., Smith S., Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002). [DOI] [PubMed] [Google Scholar]
- 91.Andersson J. L. R., Skare S., Ashburner J., How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. Neuroimage 20, 870–888 (2003). [DOI] [PubMed] [Google Scholar]
- 92.Smith S. M., et al. , Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219 (2004). [DOI] [PubMed] [Google Scholar]
- 93.Smith S. M., Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Greve D. N., Fischl B., Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48, 63–72 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jenkinson M., Smith S., A global optimisation method for robust affine registration of brain images. Med. Image Anal. 5, 143–156 (2001). [DOI] [PubMed] [Google Scholar]
- 96.Andersson J., Jenkinson M., Smith S., “Non-linear registration, aka spatial normalisation” (FMRIB Tech. Rep. TR07JA2, 2010). https://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja2/tr07ja2.pdf.
- 97.Gauthier N., Thibault P., Sullivan M. J. L., Individual and relational correlates of pain-related empathic accuracy in spouses of chronic pain patients. Clin. J. Pain 24, 669–677 (2008). [DOI] [PubMed] [Google Scholar]
- 98.Goldstein P., Weissman-Fogel I., Dumas G., Shamay-Tsoory S. G., Brain-to-brain coupling during handholding is associated with pain reduction. Proc. Natl. Acad. Sci. U.S.A. 115, E2528–E2537 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Woolrich M. W., Behrens T. E. J., Beckmann C. F., Jenkinson M., Smith S. M., Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage 21, 1732–1747 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Woolrich M. W., Ripley B. D., Brady M., Smith S. M., Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage 14, 1370–1386 (2001). [DOI] [PubMed] [Google Scholar]
- 101.Poldrack R. A., Mumford J. A., Independence in ROI analysis: Where is the voodoo? Soc. Cogn. Affect. Neurosci. 4, 208–213 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frost M. A., Goebel R., Measuring structural–functional correspondence: Spatial variability of specialised brain regions after macro-anatomical alignment. NeuroImage 59, 1369–1381 (2012). [DOI] [PubMed] [Google Scholar]
- 103.Tahmasebi A. M., et al. , Is the link between anatomical structure and function equally strong at all cognitive levels of processing? Cereb. Cortex 22, 1593–1603 (2012). [DOI] [PubMed] [Google Scholar]
- 104.Di X., Biswal B. B., Toward task connectomics: Examining whole-brain task modulated connectivity in different task domains. Cereb. Cortex 29, 1572–1583 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McLaren D. G., Ries M. L., Xu G., Johnson S. C., A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage 61, 1277–1286 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ellingsen D.-M., Anonymized data, Brain-to-brain mechanisms underlying pain empathy and social modulation of pain in the patient-clinician interaction. Open Science Framework (OSF). https://osf.io/5khjn/. Deposited 2 June 2023. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Anonymized csv, txt data have been deposited in OSF (https://osf.io/5khjn/) (106).