Abstract
Rest after traumatic brain injury (TBI) has been a part of clinical practice for more than a century but the use of rest as a treatment has ancient roots. In contemporary practice, rest recommendations have been significantly reduced but are still present. This advice to brain injured patients, on the face of it makes some logical sense but was not historically anchored in either theory or empirical data. The definition and parameters of rest have evolved over time but have encompassed recommendations including avoiding physical exercise, sensory stimulation, social contact, and even cognitive exertion. The goals and theoretical explanations for this approach have evolved and in modern conception include avoiding reinjury and reducing the metabolic demands on injured tissue. Moreover, as cellular and molecular understanding of the physiology of TBI developed, scientists and clinicians sometimes retroactively cited these new data in support of rest recommendations. Here, we trace the history of this approach and how it has been shaped by new understanding of the underlying pathology associated with brain injury.
Keywords: history, rehabilitation, rest, traumatic brain injury
Introduction
It is becoming increasingly clear that traumatic brain injury (TBI) represents an ongoing public health crisis. The vast majority of TBIs, greater than 75% by some estimates, are considered mild (often termed concussion).1 And more than 2.5 million individuals are treated in emergency departments or hospitalized for these injuries in the U.S. alone, although a substantial number of additional cases receive treatment elsewhere or are untreated.2 Fortunately, most individuals who incur a mild TBI will recover over a relatively short period of time. A substantial subset, however, can experience prolonged symptoms and debilitation.3 Critically, the available tools to identify those individuals most likely to experience poor outcomes and clinical interventions to improve outcomes are improving but incomplete.4
For a variety of historical and practical reasons that will be discussed in this article, clinicians have often prescribed prolonged rest (defined often as avoiding physical exercise, sensory stimulation, social contact, and even cognitive exertion) for the immediate period after injury. There are several putative explanations for this approach, including avoiding reinjury and reducing the metabolic demands on the injured tissue. This advice to injured patients, on the face of it makes some logical sense but was not firmly anchored in either theory or empirical data. Moreover, as we will discuss, as our modern cellular and molecular understanding of the physiology of TBI developed, scientists and clinicians sometimes retroactively cited these new data in support of rest recommendations. However, rest after brain injury is an extremely old approach that was used in antiquity and has been refined and adapted throughout the history of medicine.
Recent clinical and experimental data have demonstrated both that prolonged rest itself does little to improve functional outcomes and that the isolation and withdrawal intrinsic to this strategy can have deleterious physical, social, and educational consequences. Thus, more recent clinical practice guidelines have tempered these recommendations and now generally advocate non-contact activity (so long as it does not exacerbate symptoms) and minimal withdrawal from daily activities.5,6
Despite the relative softening of recommendations in recent years, rest was a feature of TBI medicine throughout much of recorded history and into the first part of the 21st century and remains a part of treatment algorithms. Indeed, rest recommendations so permeated both clinical thinking and popular culture that the original theoretical reasons for the existence of rest after TBI can seem obscure. The goal of this article, therefore, is not to review the current literature on rest or make clinical recommendations. There are several recent comprehensive reviews that cover that material. As researchers continue to identify the clinical and psychosocial benefits of the opposite strategies including exercise, cognitive stimulation, and social engagement, and rest recommendations continue to evolve, it is worth taking time to consider how and why the original recommendations developed and whether they have a place in current thinking about optimizing recovery from traumatic brain injury.
Historical Perspective on Rest as Treatment for TBI
Before a discussion of the historical roots of rest as a treatment strategy for mild TBI, it is important to briefly review the development of mild TBI/concussion as an independent diagnostic entity separate from more severe brain injuries. This is of critical importance to the history of rest as a treatment because individuals with severe head injuries throughout most of history were very likely to die and, in any case, would have been unlikely to be able to do anything beyond rest. In contrast, milder head injuries, particularly those that occurred in the absence of skull fractures, received comparatively minimal clinical attention for much of recorded medical history. Thus, it required the development of a clinical construct where individuals were at least theoretically capable of returning to their regular activities for a recommendation for rest to make any sort of logical sense (see the Supplementary Appendix for expanded discussion of the development of a concept of mild traumatic brain injury). Moreover, the conceptualization of the underlying pathophysiology and thus the theories driving treatment strategies have also changed dramatically. Finally, the prescribed rest approach is inextricably tied to the long running argument as to whether prolonged mild traumatic brain injury symptoms represent “organic” brain dysfunction or are the result of malingering and/or seeking financial benefit and avoidance of work.
Rest as a therapeutic strategy, in general, has ancient origins. Hippocrates in the Aphorisms, says “in every movement of the body, whenever one begins to endure pain, it will be relieved by rest.” There is little evidence, however, that ancient civilizations used bed rest as a therapeutic strategy for TBI specifically. Moreover, a distinction has to be drawn between patients that were unconscious or otherwise forced by medical necessity to rest. The subject of this paper is the medical recommendation for rest as a therapeutic intervention for TBI that was given to individuals who were physically capable of activity. Given that for much of human history milder head injuries received comparatively little attention in the surviving medical records, it is difficult to know whether concussed individuals would have even been considered in need of medical treatment at all, let alone bed rest.
For more severely injured patients, the effective treatments available were extremely limited beyond supportive care. A physician writing in the 19th century took issue with the Hippocratic point that individuals with brain injuries could not recover. He made the slightly underwhelming argument that in the preceding 1200 years of recorded medical history (in Western Europe) that at least 100 patients had survived brain injuries.7 The argument was that while it was still extremely unlikely to survive it was at least theoretically possible. One additional point to consider is that what is now considered “mild” TBI produced much higher chances of morbidity and mortality than in modern times given the sometimes-aggressive surgical interventions, bloodletting, and lack of understanding of infectious processes, among other iatrogenic processes.
Some insight into ancient thinking about the role of rest in disease processes can be gleaned from examining the medical recommendations regarding exercise. Ancient theories of health that postulated a balance among competing humors allowed for exercise to modify the balance among them.8 For instance, the ancient Indian physician, Susruta (active around 600 BCE)—often credited with inventing plastic surgery—was an advocate of the tridosa doctrine, which held that health was the result of balance among the dosas (humors) vayu, pitta, and kapha, and that disease resulted from imbalance among these. His book the Sushruta Samhita is the oldest surviving written example of physician prescribing exercise to his patients. Sushruta's recommended that exercise be taken daily but only to around half that individual's capacity lest it prove fatal.9 He argued that exercise reduced the kapha dosa, helping to restore balance. In the Mediterranean, Hippocrates of Kos was a supporter of the Aristotelian humor theory, and an advocate for exercise as a component of both health and as a treatment for disease. He famously wrote that “eating alone will not keep a man well; he must take exercise.”
The classical physician that dominated Western medical thought for more than a millennium, however, was the Roman physician Galen. Claudius Galenus, who was active in the late second and early third centuries CE, was an admirer of Hippocrates and described both “natural” and critically “non-natural” contributors to health and disease. His six non-naturals included the air and environment, diet, sleep and wake, motion and rest, retention and evacuation, and emotions. Balance among these factors was necessary for the maintenance (or recovery) of health.10 In Art of Medicine, Galen wrote “when, for example, the body is in need of motion, exercise is healthy and rest morbid; when it is in need of a break, rest is healthy and exercise morbid.” The Galenic conception of humoral balance persisted for more than a millennium in Western medical thought and in some forms for much longer.
One area of classical antiquity where treatment of TBI received significant attention was on the battlefield. Roman soldiers who suffered head injuries in battle were transported to field hospitals by medical orderlies and triaged.11 Moderate cases were treated first while mildly and terminally injured soldiers were mostly given supportive care. There was a focus on hygiene in medical establishments with separation of the ill from the injured, sterilization of instruments, and cleaning of wounds with disinfectants. Importantly, bed rest and observation were a component of the brain injury recovery protocol (along with dietary modifications and herbal medicines). This approach during the high period of the Roman Empire was markedly more sophisticated than brain injury care in the rest of Roman society or indeed in other civilian or military medical establishments until the 19th century.11
Prior to the 1860s, there was comparatively little bed rest prescribed among patients capable of engaging in activities of daily living. The term was not even found in the indexes of several large comprehensive medical texts published between 1840 and 1860.12 There were several reasons for this. First the economic conditions for most individuals did not permit withdrawal from work and an illness that required bedrest could thus produce calamitous results. It appears more likely, at least among the lower social classes, that individuals would conceal their illnesses rather than risk losing employment.13,14 Second, there was a significant stigma associated with an inability to work that resulted in part from religious doctrine and the association between the domestic space and femininity. Thus, there was a significant association between being bedridden and emasculation.15,16 Overall rest was not a common feature of traumatic brain injury therapy (or indeed medicine more broadly) before the mid-19th century.
Establishment of Rest as Medical Treatment for TBI
From the classical period and into the Renaissance (and in some cases well beyond), treatments of brain injuries were largely based around Galenic practices including bloodletting, cupping, purgatives, modulation of diet, and in some cases surgical interventions directed at the skull. The full development of rest as a therapeutic strategy in TBI required several developments in medical theory and societal structure. First, there needed to be the development of the concept of a mild TBI that produced persistent impairments in the function of the nervous system, even in the absence of overt damage to the skull and underlying tissue. Second, there had to exist a social structure and sufficient resources for individuals to be able to afford prolonged absence from work and domestic responsibilities.
Therefore, in the early 1860s, when John Hilton, a surgeon at Guy's hospital in London (and surgeon extraordinary to Queen Victoria), delivered his series of lectures on the use of rest as a therapeutic strategy to the English Royal College of Surgeons it caused a sea-change in the treatment of traumatic brain injury (among many other conditions). Hilton made a series of arguments for the use of rest based on both his detailed understanding of anatomy and his apparent belief that contemporary surgical and medical interventions often did more harm than good. Specifically, he argued that: 1) cycles of rest and growth were critical to normal development in both plants and animals; 2) animals would seek rest after injury; 3) repair was repetition of growth so that the elements necessary for optimal development were also those required for repair after injury; 4) organs physically swell when they are more physiologically active and that these same organs are encased within membranes that help to return them to their prior “resting” size; 5) the cerebrospinal fluid and ventricles served a similar function to elastic capsules on other organs, helping to reduce the turgescence of brain tissue when at rest; 6) individuals who suffer a head injury and exhibit persistent symptoms must have something more than temporary vascular dysfunction (i.e., that the brain itself was in a state of “collapse” or “shock”). Here, Hilton draws a direct comparison between a bruise of peripheral tissue and concussion. He argues that the contemporary practice of immobilizing external injuries should be directly applied to the treatment of concussion; 7) treatments of the brain injured that were stimulating (he cites brandy or ammonia) were necessarily harmful; 8) Hilton thus argues that all “stimulating” stimuli including animal food, excess temperature, and physical, cognitive or emotional exertions, must be avoided particularly if they cause any pain; 9) the length of rest should be both proportional to the severity of the injury and personalized based on the recovery of the individual; and finally 10) if this course of action is not taken, that there is a risk for “obvious and serious chronic lesions, such as failure of mental power, softening, chronic inflammation, extravasation of blood etc.”17
The impact of this work on medical practice, both related to TBI and in other specialties, can hardly be overstated. Prior to the 1860s, surgical manuals describe brain injury care and typically recommend diet, bleeding, and other contemporary treatments but tend not to specifically discuss how recovery should be managed.18-20 Following the Hilton lectures and well into the 21st century rest was a nearly universal feature of medical practice guidelines for TBI. The specific guidelines could vary between practitioners, with some recommending a week of quiet for concussion21 while others recommended only a few days followed by a graded return to activity,22 and some suggesting 2 weeks or more.23-25 The early-mid 20th century physicians often included in these recommendations ideas about the number and relationships of visitors, the position of the patient in bed, and at times the use of sedatives and other “rest-promoting agents.” Moreover, it was not uncommon to require “supervision” of the bed rest to ensure that the patient “does not have bathroom privileges.”26 To be sure, there were competing voices among practitioners that derided rest as the conservative and less proactive approach to the treatment of TBI. However, even those that sought to treat the patients more aggressively (surgically or with other medical interventions) acknowledged that their preferred treatments should occur in addition to rest.27-29
Limitations and Backlash
The late 19th and early 20th century represented a time of rapid industrialization, which brought about several societal changes with important implications for the conceptualization and management of brain injuries. With the great increase in heavy industry, railroads, and automobiles, the incidence of workplace and transportation-related injuries, many of which involved much greater force than civilians had previously experienced, also rose significantly. During this period, workmen's compensation laws began to be enacted in Western Europe and the U.S. At the same time, neurobiological science was wrestling with the long running “organicity” argument associated with mental illness in general and TBI in particular—that is, the question of whether a diseased mind could exist in a healthy brain.
What is critical for the current topic, however, is that the increase in industrial and transportation-related injuries also produced increases in litigation.30 The combination of individuals with injuries that could not be verified by extant medical examination or testing and a vested financial interest in downplaying the long-term sequela of brain injuries led to a long-term disagreement within medical circles as to whether long-term post-injury symptoms represented “organic” damage to the brain on the one extreme, or frank malingering on the other.31-33 Between the two extremes, other theorists staked out middle grounds, suggesting that stress induced by the litigation, unconscious malingering, or the amplification of a pre-injury predisposition to mental illness were responsible for post-injury dysfunction.32,34–38 This argument extended through the world wars and came to include the possibility that individuals were either inventing symptoms to avoid military service altogether or exaggerating them after a military injury. This situation was superimposed on the then current opinions about class and gender.
Then, as now, people of lower socio-economic classes were much more likely to suffer TBIs, particularly in industrial settings.39,40 Accidents and the resulting injuries were often blamed on perceived shortcomings of the injured. Moreover, as poverty was considered the result of a moral failing by the poor, those individuals that suffered head injuries were ipso facto believed to be more likely to fake their symptoms to gain material advantage.41 All of this added up to a pressure for physicians to downplay chronic symptoms of brain injury and in some cases to avoid using rest as a treatment. Finally, the relative lack of social welfare system during the early 20th century forced many who individuals that suffered a brain injury to forego rest in favor of work and domestic responsibilities.
Rest was strongly tied up in the concerns about long-term symptoms after brain injury. The terms used included (but were not limited to) accident neurosis, compensation neurosis, traumatic encephalitis, traumatic neurosis, and traumatic neurasthenia among many others.42 The concern about chronic injury sequela is much older, having been first raised by Depuytren and Cooper in the early 19th century.42 However, the publication by John Erichsen of a series of lectures in 1866 coined the term railway spine.43 This disease, the symptoms of which included many classic concussion symptoms, was thought to arise from “molecular disarrangement” of the spinal cord induced by rail travel. Later commentators would note that the name seemed so inappropriate to the condition as to invalidate its existence.
The cases reported recently in the Journal by Dr. J. J. Putnam, and the paper in a late issue by Dr. Walton, point to the reality of a set of symptoms induced by traumatism which corresponds well with those hitherto termed spinal concussion, a name so misleading that many accurate observers through the influence of the name alone have been induced to deny the existence of what the name covers.44
These conditions, which reflected strong theoretical and medico-legal distinctions at the time, steadily evolved over the course of the 20th century and were fiercely debated, but largely contained the symptoms associated with modern conceptions of post-concussion syndromes and post-traumatic stress disorders. Of great concern to mid-20th century physicians was that patients be convinced very early in their treatment that their injuries were mild and that they would fully recover. Moreover, any symptoms that they experienced were normal and would soon subside. Thus the use of rest (and the subsequent out-patient period before the individual returned to work), while valuable to aid the recovery process had to be carefully balanced against the risk that resting too long would cause a neurosis.45
According to Guttmann, a mid-20th century psychiatrist, “Reaction to treatment (or mismanagement), in fact, may be classified as a special type of accident neurosis following head injury. Prolonged bed rest is a factor strongly suggestive of seriousness of the injury; in particular, if the patient feels subjectively well, he is liable to conclude, when kept in bed for several weeks, that the doctor must fear serious consequences, and this fear is easily transferred to the patient himself. Such apprehensions, or rather, misapprehensions, are often expressed to the patient, who cannot fail to be impressed and to start watching for after-effects.”46
On the other hand, the treatment for prolonged post-injury symptoms was often rest. This rest was conceived a bit differently than the immediate post-injury rest as it emphasized the isolation, particularly from troublesome relatives that could be reinforcing the psychopathology.47,48
The tendency for bed rest to dominate medical practice in other domains had been firmly established by the time of the World War II. It was not uncommon, for instance, for individuals recovering from heart attacks to be ordered to bed for several weeks.49,50 Tuberculosis was treated with long rest periods, even including chest casts to restrict (and thus rest) damaged lungs.51 Similar approaches were taken for orthopedic injuries and after surgery. In some cases, patients recovering from hernia surgery would be wrapped in plaster casts to completely immobilize them.52 Doctors who suggested that patients be allowed even modest physical activity were considered radical.53 A few events helped to loosen this domination of the bed rest approach. The use and benefit of early ambulation after abdominal surgery had been suggested by Emil Ries,54 a recommendation that was not fully appreciated in its time.53 However, in the 1940s the combination of limited beds available for convalescence by servicemen injured in the war and a series of publications by Daniel Leithauser in the 1940s and 1950s showing that early ambulation after surgery was both safe and actually improved the outcomes from minor surgical procedures55-57 helped begin to loosen the domination of prolonged bed rest. Additionally, there was a growing body of work that demonstrated that there were significant physiological risks to bed rest that included, but were not limited to thrombosis, congestive heart failure, and bed sores.58-60 Norman Browse's writing in the mid-1960s would argue that Hilton's work had been misinterpreted by the medical community and that although bedrest had a place in clinical practice that it was not the only treatment available and could be overdone.61 He also made the interesting argument that bed rest had come to so dominate medical practice that hospitals were (and still are) classified by the number of beds that they possess. Even the term “clinical” derives from the Latin (and Greek) terms for bed. Browse suggests that medicine should be “cathedral” (related to a chair or throne) rather than clinical, an idea that has thus far failed to catch on.
Thus, by the second half of the 20th century, rest had fallen from its perch as the ubiquitous frontline approach for treatments across medical practice. What is striking, however, is that to a very great extent a similar process did not occur in the field of TBI medicine where rest recommendations persisted far longer.
Into the Modern Era
Despite the gradual waning of rest as a ubiquitous frontline strategy in medicine generally,62 clinical best practices for brain injury have continued to include rest well into the 21st century.63 There were some notable published treatment paradigms in the aftermath of World War II that advocated for shorter periods of bed rest, a result of apparently anecdotal findings that wartime shortages of manpower and hospital beds had necessitated shorter periods of rest without adversely affecting treatment outcomes.64,65 However, systematic studies of rest as a strategy did not occur until later in the century. As far back as the 1950s (at least in English, as several earlier studies were cited in Scandinavian languages), studies began to compare treatment regimens that included bed rest. Several key points about these early studies must be made. First, they typically included patients across a range of injury severities; second, they often compared the outcomes using their treatment protocol to historical findings in other studies (rather than directly comparing alternative approaches); and third, bed rest comparisons were often of degree and length rather than asking whether any rest was necessary. For instance, in a 1957 study, hospitalized patients with a concussion (without evidence of focal lesions or cerebral laceration) but ranging from minor to severe (based on the length of unconsciousness) were confined to bed for 2-6 days and compared with historical datasets with 2-3 weeks of bed rest.66 They concluded that shorter periods of bed rest did not result in worsened outcomes. A study performed in the mid-1970s at the University of Groningen compared outcomes before and after a shift in treatment paradigm. Individuals treated in 1974-1975 were given minimal information about their injuries and told to rest in bed “as long as necessary” but the length was not systematically controlled. After 1977 patients were given explicit information as to the nature of their injuries and told to rest in bed for 1 week before gradually increasing their activity.67 Overall, there were no marked differences between the treatment paradigms in terms of outcomes. That paper is notable because it reported the length of time patients remained on bed rest, and even with the updated treatment regimen instituted in the late 1970s, more than 70% of individuals remained in bed for between 1 and 4 weeks, with a substantial minority (around 10%) for whom bed rest lasted more than 4 weeks. A 2001 survey of physicians treating mild TBI in Europe reported that more than 40% recommended full bed rest lasting from 1-14 days.63
Changing Justifications
As the nature and conceptualization of rest as treatment for traumatic brain injury has continued to evolve, so has the rationale for use of rest as a rehabilitative approach. Hilton made the explicit argument that resting was appropriate for injuries to the periphery, and that a similar logic should apply to insults to the brain (see “Establishment of Rest as Medical Treatment for TBI” above); however, as the science progressed the stated reasons for the use of rest have changed. For much of the late 19th and early 20th century, focus was around acute recovery (e.g., the avoidance of brain softening) and preventing the development of persistent post-concussion symptoms. As the 20th century progressed and policymakers and clinicians became increasingly concerned about the long-term sequalae of (especially repeated) head injuries, management of sports-related injuries became part of the broader conversation, with implications for rest.68-70 Rest as a therapeutic approach became linked to the related but distinct concept of return-to-play. If individuals were held out of contact sports after suffering TBI, then the chances of secondary or repeated injuries were necessarily reduced.71
Many studies reported that exertion either cognitively or physically could acutely induce worsening of TBI symptoms.72-74 Thus, the concept of rest most often came to include a goal that individuals should be symptom-free with exertion before resuming athletic or academic pursuits. There was an implicit assumption that the reduction in symptom exacerbation with exertion represented a key milestone in recovery and could be used to assess progress and to guide activity recommendations.
Additionally, new physiological data on the effects of exertion on intracranial pressure regulation and the metabolic consequences of brain injuries would be used to bolster the rest recommendations. For instance, in 2001, an article was published describing the neurometabolic dysfunction associated with TBI and laid out the concept that injury induces a cascade of physiological and pathological events that must resolve before optimal function can be restored, and thereby vulnerability to repeated injury would lessen. This hugely influential article (cited more than 1800 times as of 2022 and updated in 2014 with another 1100+ citations) has greatly impacted the conversation around concussion management.75,76 The data discussed in these papers focus attention on neurometabolic function and raise the possibility that exercise and cognitive exertion could imperil brain recovery because placing increased energetic demands is hazardous when metabolic physiology is impaired.77 Moreover, there were reports that injury could decouple cerebral blood flow from energetic needs78 and thus that the elevated energetic demands associated with exercise could functionally deprive the brain of critical energy supplies and that physical exertion, as simulated by the Valsalva maneuver significantly elevated intracranial pressure.79
A series of experimental findings published in the early 2000s on a rat model of voluntary exercise following fluid percussion injury also bolstered the rest recommendations. Injured rats that were allowed access to a running wheel in the immediate post-injury period exhibited impairments in the expression of survival- and plasticity-related proteins, and impairments in spatial learning and memory performance.80,81 Additionally, access to running wheels immediately after brain injury could drive increases in hypothalamic-pituitary-adrenal-mediated stress responses, which could be deleterious to recovery.82 However, emerging experimental findings on the benefits of physical exercise after brain injury suggest that with adjustments in the timing and intensity of exercise, both the pathology and functional recovery after TBI is improved relative to non-exercised animals.81,83–87
Taken together, the rapid advances in the understanding of the biology of traumatic brain injuries that occurred in the late 20th and early 21st centuries led clinicians and scientists to both revise and reinterpret the rest recommendations that had been a part of treatment protocols for generations. Although rest recommendations were increasingly moderated, experimental data could be used to explain the benefits of the approach.
Prognostic Impact of Rest as Treatment
While the rationale for rest after TBI has evolved, the use of physical and/or cognitive rest until symptom resolution has persisted, albeit the basis for determining rest duration and criteria for symptom resolution remain inconsistent. Critically however, we now have a growing body of research empirically testing and challenging the utility of rest (particularly prolonged rest) as a reliable rehabilitative approach to mild TBI treatment. An early study compared brain injury patients who were provided with ‘routine’ treatment (not encouraged to be active) to those who were provided with “active” treatment (encouraged to be mobile as soon as possible and provided with physiotherapy) for approximately a 1-week hospital stay. Patients who underwent “active” treatment were less likely to experience post-traumatic amnesia, returned to work sooner, and were less likely to report lingering symptoms during the 1-year follow-up.88
To our knowledge the first truly randomized control trial for bed rest after TBI was conducted at the Maastricht University Hospital in the Netherlands. Individuals who suffered a mild uncomplicated TBI were randomly assigned to one of two conditions, complete bedrest for the first 6 days followed by increasing mobilization on Days 6-10 or were instructed to move from the first day after injury and to spend no more than 4 h in bed during daylight hours. Individuals instructed to rest in bed spent more than twice as many daylight hours in bed as the individuals allowed to mobilize quickly; however, these individuals reported more difficulty complying with the advice than did those who were mobilized. This study further reported that dizziness and other somatic complaints were reduced during the first few days of bedrest; however, at later follow-ups, there were no overall benefits of bed rest during the early post-injury period and concluded overall that bed rest was neither necessary nor beneficial.89
A key component of rest intervention is withdrawal from social and educational activities. As a result, the perceived isolation has been shown to both impair recovery and have a negative impact on mental health, especially in adolescents. In recent trials, adolescent concussion patients who were prescribed rest before returning to school, work, or physical activity experienced a greater number of post-concussive symptoms for a longer duration compared with concussion patients who were not advised to rest.90,91 A published review of concussion online forums for athletes revealed that restricted activities exacerbate feelings of loneliness and social isolation, which can often be experienced as perceived lack of support and contributes to development of depression, poor coping mechanisms, and contemplation of suicide.92
Individuals who sustain a mild TBI and are then prescribed prolonged rest also run the risk of experiencing deconditioning (reduced cardiovascular, pulmonary, and muscle function), which can occur after as little as 2 to 3 days of inactivity.93 Indeed, even relatively short periods of restriction of physical activity exacerbate TBI-induced impairments in cerebral vasoreactivity and blood flow regulation and contribute to persisting post-concussive symptoms.94-96 The physical and emotional consequences of bed rest are often misattributed to the concussion itself and promote further experiences of isolation as concussed athletes need time to regain fitness.97 In contrast, a randomized controlled trial comparing concussed athletes who were prescribed rest, subthreshold aerobic exercise, or gentle stretching reveal that active exercise in the week following injury reduces both symptom severity and incidence of delayed recovery.98
The Current Approach
The definition of rest has evolved and is necessarily vastly different from what Hilton and his contemporaries would have recommended, much less what would have been possible in ancient societies. The term rest, in the modern conception, can thus be further divided into complete rest (dark quiet rooms), cognitive rest, physical rest, and the restrictions of electronic devices and screen time. Moreover, all of these approaches are (or at least can be) distinct from the avoidance of contact sports or other activities that could lead to repeated injuries.
As the evidence for deleterious consequences of lengthy rest has continued to mount and the potential lost opportunities for beneficial effects of exercise99 and other forms of stimulation has emerged the general trend has been to limit, but not eliminate the recommendation for rest.100 The trend in current practice guidelines has been to decrease the duration of recommended rest, reduce the requirement that symptoms must resolve before increasing activity and to acknowledge that the evidence for activity restriction is equivocal.5,6,101 There is also a push to include exercise prescription as safe and beneficial to recovery (Leddy and colleagues, 2019). Further a tendency exists to treat younger patients more ‘conservatively’ (i.e., more rest).102-104 Moreover, it is increasingly clear that even relatively mild activity restrictions are poorly implemented105,106 and adherence is relatively low, particularly among children.107,108 Despite these changes, many patients are still being told to rest longer than practice recommendations advocate and thus continued education of TBI clinicians remains paramount.109
The Future
Clinical recommendations and treatment paradigms are not static and evolve both theoretically and practically over time. Rest recommendations, like many long-standing medical treatments, have been subject to waxing and waning popularity over time. While there is little evidence that prolonged bed rest produces better outcomes than other approaches, there is still much to understand about the approach that will produce the best possible outcome for any individual that suffers a brain injury. It is likely that some amount of rest coupled to exercise will be beneficial to recovery. However, it is equally possible that a threshold exists wherein exercise can be too intense and imperil recovery and this is likely to vary significantly with a whole host of variables including age, comorbidities, sex, injury type and severity, and level of pre-injury fitness among many others. Thus, future research should be dedicated to individualizing and titrating levels of exercise and rest and developing feedback mechanisms to inform patients and their doctors as to whether their activity levels are beneficial.
There are also key unanswered questions with regard to screen usage in those recovering from a TBI. This is a critical question given the ubiquity of devices in the modern world and that restrictions on their usage can exacerbate feelings of isolation, particularly among younger individuals. However, screen usage could potentially exacerbate symptoms via mechanisms including eye strain, induction of migraines, and other unknown processes. Moreover, if screen time displaces physical activity, it could deprive individuals of the beneficial effects of exercise.110
There is also much that we do not understand as to how rest, exercise, and related variables are mechanistically translated into injury outcomes. If we can understand the mechanisms responsible for both the beneficial and deleterious effects of activity, it will likely help both optimize recovery and prevent the potential exacerbations of outcomes.
Conclusions
Rest after traumatic brain injury has a very long history and has been a mainstay of treatment paradigms in different forms for millennia. For much of human history, traumatic brain injuries have fallen into two broad categories, mild enough to be essentially ignored or severe enough to be potentially fatal. As our theoretical understanding of TBI has evolved, alongside other societal advances, it has become possible to help traumatic brain-injured individuals across the severity spectrum.
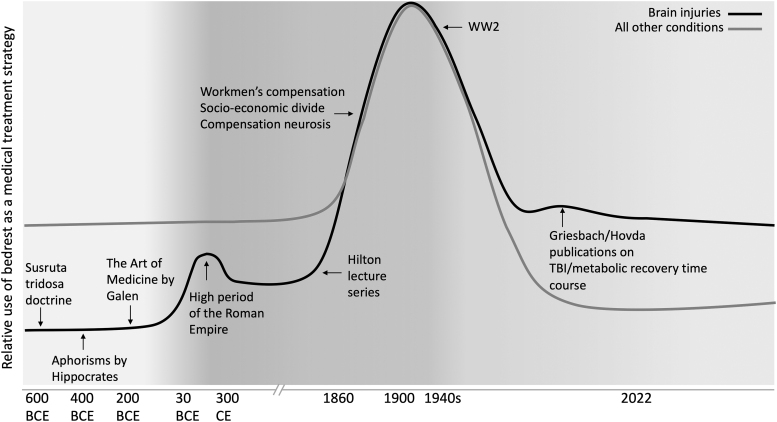
The modern conception of individuals who were physically capable of activity nevertheless being instructed to rest originated with the ideas of John Hilton in the 1860s. His ideas dominated clinical practice in neurological injury and many other areas of medicine for most of the next century. However, other areas of medicine began to move away from strict rest as a frontline treatment as both the direct costs of prolonged rest and the benefits of activity became clearer. Rest recommendations for brain injury persisted (and continue) for much longer than in other areas of medicine (with some notable exceptions like high-risk pregnancy where bed rest persisted; see Fig.1 for a summary of major events in this story). The reasons for this likely involve that: 1) exertion can temporarily exacerbate neurological symptoms and thus resting did avoid this; 2) few clinical interventions were (or are) broadly effective at preventing long-term negative consequences of brain injury and so rest was considered a safe and potentially beneficial strategy; and 3) medical practice is somewhat conservative such that old ideas can persist despite a lack of evidence for their benefit.
FIG. 1.
Major events and trends in the historical use of rest as a treatment strategy for brain injuries and for other medical conditions.
As scientific understanding of brain injury pathophysiology changed so did the reasons stated for recommending rest until settling on the modern views that rest has the benefit of reducing the likelihood of reinjury, minimizing metabolic demands on the tissues, and preventing symptom exacerbation. This approach is not without costs and science has understood for decades that prolonged bed rest was medically and psychologically hazardous. Moreover, it does not allow the potentially beneficial effects of exercise and other enriching activities. Further, the definition of rest has changed in duration and degree and come to encompass other modern ideas like screen time. Whether the future will see the complete removal of rest recommendations remains unknown but modern clinicians should be aware that these choices have been made by their predecessors over a very long period of time and that the original conception of rest was made at a time when our understanding of the biology of head injury was fundamentally different from what it is now.
Supplementary Material
Acknowledgments
The authors thank John Corrigan for his insightful comments on an earlier version of this manuscript.
Authors' Contributions
Zachary Weil: conceptualization, writing—original draft. Julia Ivey: writing—original Draft. Kate Karelina: conceptualization, writing—review and editing.
Funding Information
Preparation of this manuscript supported by National Institutes of Health Institute for General Medical Sciences, Award Number 2U54GM104942-03.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Wright DW, Kellermann A, McGuire LC, et al. CDC grand rounds: reducing severe traumatic brain injury in the United States. Morbid Mortal Wkly Rep 2013;62(27):549. [PMC free article] [PubMed] [Google Scholar]
- 2. Voss JD, Connolly J, Schwab KA, et al. Update on the epidemiology of concussion/mild traumatic brain injury. Curr Pain Headache Rep 2015;19(7):1–8; doi: 10.1007/s11916-015-0506-z [DOI] [PubMed] [Google Scholar]
- 3. Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma 2010;27(8):1529–1540; doi: 10.1089/neu.2010.1358 [DOI] [PubMed] [Google Scholar]
- 4. Korley FK, Jain S, Sun X, et al. Prognostic value of day-of-injury plasma GFAP and UCH-L1 concentrations for predicting functional recovery after traumatic brain injury in patients from the US TRACK-TBI cohort: an observational cohort study. Lancet Neurol 2022;21(9):803–813; doi: 10.1016/S1474-4422(22)00256-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport—the 5th International Conference on Concussion in Sport held in Berlin, October 2016. Br J Sports Med 2017;51(11):838–847; doi: 10.1136/bjsports-2017-097699 [DOI] [PubMed] [Google Scholar]
- 6. DeMatteo C, Randall S, Falla K, et al. Concussion management for children has changed: new pediatric protocols using the latest evidence. Clin Pediatr 2020;59(1):5–20; doi: 10.1177/0009922819879457 [DOI] [PubMed] [Google Scholar]
- 7. Yonge J. Wounds of the Brain Proved Curable. London; 1862. [Google Scholar]
- 8. Tipton CM. The history of “Exercise Is Medicine” in ancient civilizations. Adv Physiol Educ 2014;38(2):109-117; doi: 10.1152/advan.00136.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Susruta M. Susruta Samhita, Part 2. Varanasi, Edition. Chaukhambha Orientalia; Maidagin, Varanasi, India; Reprint 2006;156–157. [Google Scholar]
- 10. Berryman JW. Motion and rest: Galen on exercise and health. Lancet 2012;380(9838):210–201; doi: 10.1016/s0140-6736(12)61205-7 [DOI] [PubMed] [Google Scholar]
- 11. Belfiglio VJ, Sullivant SI. Roman Military Medicine: Survival in the Modern Wilderness. Cambridge Scholars Publishing: Newcastle upon Tyne, U.K.; 2019. [Google Scholar]
- 12. Spender JK. On movement as a therapeutic agent. BMJ 1880;2(1023):205–207; doi: 10.1136/bmj.2.1023.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Withey A. Sickness experience and the “sick role.” In: Physick and the Family: Health, Medicine and Care in Wales, 1600-1750. Manchester University Press: Manchester U.K.; 2011. [Google Scholar]
- 14. Beier LM. Sufferers and Healers: The Experience of Illness in Seventeenth-Century England. Routledge: Oxfordshire, U.K.; 2015. [Google Scholar]
- 15. Shepard A. Meanings of Manhood in Early Modern England. Oxford University Press: Oxford, U.K.; 2003. [Google Scholar]
- 16. Turner DM. Disability in eighteenth-century England: Imagining physical impairment. Routledge: Oxfordshire, U.K.; 2012. [Google Scholar]
- 17. Hilton J. On Rest and Pain: A Course of Lectures on the Influence of Mechanical and Physiological Rest in the Treatment of Accidents and Surgical Diseases, and the Diagnostic Value of Pain. Nabu Press (January 6, 2010): Berlin, Germany; 1880. [Google Scholar]
- 18. Pott P. Observations on the Nature and Consequences of Those Injuries to which the Head is Liable from External Violence: To which are Added, Some Few General Remarks on Fractures and Dislocation (Classic Reprint). Forgotten Books: London, U.K.: 1768. [Google Scholar]
- 19. Abernethy J. Surgical Observations on Injuries of the Head. Longman, Hurst, Rees, Orme, Brown, & Green: Harlow, U.K.; 1825. [Google Scholar]
- 20. Brodie BC. Pathological and surgical observations relating to Injuries of the Brain: Part I. Med Chir Trans 1828;14(Pt 2):325-423; doi: 10.1177/09595287280140p202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheyne WW. A Manual of Surgical Treatment. Lea Brothers & Company: 1903. [Google Scholar]
- 22. Symonds C. Observations on the differential diagnosis and treatment of cerebral states consequent upon head injuries. BMJ 1928;2(3540):829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferguson FR, Liversedge LA. Modern views on concussion. Practitioner 1946;156:26–32. [PubMed] [Google Scholar]
- 24. Ramsay J. Nervous Disorder after Injury. BMJ 1939;2(4102):385–390; doi: 10.1136/bmj.2.4102.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mixter WJ. Treatment of head injuries. J Natl Med Assoc 1926;18(3):119–123. [PMC free article] [PubMed] [Google Scholar]
- 26. Lehmann PO, Elvidge AR. Post-traumatic headache and dizziness: (A Study of the Causal Injury). Can Med Assoc J 1942;47(3):197–201. [PMC free article] [PubMed] [Google Scholar]
- 27. Maclaire AS. The treatment of acute traumatic craniocerebral injuries. JAMA 1926;86(10):670–673; doi: 10.1097/00000658-192606000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dowman CE. Management of head injuries with real or potential brain damage: with special reference to the value of saturated solutions of magnesium sulphate and sodium chlorid. JAMA 1922;79(27):2212–2214. [Google Scholar]
- 29. Munro D. The diagnosis, treatment and immediate prognosis of cerebral trauma. New Engl J Med 1934;210(6):287–294. [Google Scholar]
- 30. Casper ST. Concussion: a history of science and medicine, 1870-2005. Headache 2018;58(6):795–810; doi: 10.1111/head.13288 [DOI] [PubMed] [Google Scholar]
- 31. Russell WR. Cerebral involvement in head injury 1: a study based on the examination of two hundred cases. Brain 1932;55(4):549–603; doi: 10.1093/brain/55.4.549 [DOI] [Google Scholar]
- 32. Miller H. Accident neurosis. BMJ 1961;1(5230):919–925; doi: 10.1136/bmj.1.5230.919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Osnato M, Giliberti V. Postconcussion neurosis-traumatic encephalitis: a conception of postconcussion phenomena. Arch Neurol Psychiatry 1927;18(2):181–214. [Google Scholar]
- 34. Strauss I, Savitsky N. Head injury: neurologic and psychiatric aspects. Arch Neurol Psychiatry 1934;31(5):893–955. [Google Scholar]
- 35. Bramwell B. Malingering, valetudinarianism, and their prevention. BMJ 1913; doi: 10.1136/bmj.1.2729.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hall GW, MacKay R P. The posttraumatic neuroses. JAMA 1934;102(7):510–513; doi: 10.1001/jama.1934.02750070008002 [DOI] [Google Scholar]
- 37. Miller H, Cartlidge N. Simulation and malingering after injuries to the brain and spinal cord. Lancet 1972;299(7750):580–585; doi: 10.1016/s0140-6736(72)90368-6 [DOI] [PubMed] [Google Scholar]
- 38. Zenner P. Traumatic Neurosis. JAMA 1907;XLVIII(15): [Google Scholar]
- 39. Kraus JF, Fife D, Ramstein K, et al. The relationship of family income to the incidence, external causes, and outcomes of serious brain injury, San Diego County, California. Am J Public Health 1986;76(11):1345–01347; doi: 10.2105/AJPH.76.11.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bruns JJ, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia 2003;44(s10):2–10; doi: 10.1046/j.1528-1157.44.s10.3.x [DOI] [PubMed] [Google Scholar]
- 41. Casper ST. The intertwined history of malingering and brain injury: an argument for structural competency in traumatic brain injury. J Law Med Ethics 2021;49(3):365–371; doi: 10.1017/jme.2021.55 [DOI] [PubMed] [Google Scholar]
- 42. Evans RW. The postconcussion syndrome: 130 years of controversy. Semin Neurol 1994;14(1):32–39; doi: 10.1055/s-2008-1041056 [DOI] [PubMed] [Google Scholar]
- 43. Erichsen JE. On railway and other injuries of the nervous system. Br Foreign Med Chir Rev 1867;40(79):102–109. [PMC free article] [PubMed] [Google Scholar]
- 44. Smith, JVC. The Boston Medical and Surgical Journal, 1840, Vol. 21 (Classic Reprint) Hardcover—27 Dec. 2018. Forgotten Books: London, U.K.0; 2018. [Google Scholar]
- 45. Symonds C, Jefferson G. The treatment of head injuries. BMJ 1935;2(3901):677; doi: 10.1136/bmj.2.3901.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guttmann E. Late effects of closed head injuries: psychiatric observations. J Ment Sci 1946;92(386):1–18; doi: 10.1192/bjp.92.386.NP [DOI] [PubMed] [Google Scholar]
- 47. Sinkler W. Prognosis and treatment of the traumatic neuroses. JAMA 1900;35(12):735. [Google Scholar]
- 48. Caplan EM. Trains, brains, and sprains: railway spine and the origins of psychoneuroses. Bull Hist Med 1995;69(3):387–419. [PubMed] [Google Scholar]
- 49. Irvin CW Jr., Burgess AM Jr. The abuse of bed rest in the treatment of myocardial infarction. N Engl J Med 1950;243(13):486–489; doi: 10.1056/NEJM195009282431304 [DOI] [PubMed] [Google Scholar]
- 50. Herrick JB. Clinical features of sudden obstruction of the coronary arteries. JAMA 1912;LIX(23):2015–2022; doi: 10.1001/jama.1912.04270120001001 [DOI] [PubMed] [Google Scholar]
- 51. Levitt N. The plaster cast in the treatment of pulmonary tuberculosis. Am Rev Tuberculosis 1922;6(5):410–416. [Google Scholar]
- 52. Bloodgood JC. Operations on 459 cases of hernia in the Johns Hopkins Hospital from June, 1889 to January, 1899. Friedenwald Company: 1899. [Google Scholar]
- 53. Brieger GH. Early ambulation. A study in the history of surgery. Annals of surgery 1983;197(4):443; doi: 10.1097/00000658-198304000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ries E. Some radical changes in the after-treatment of celiotomy cases. JAMA 1899;33(8):454–456. [Google Scholar]
- 55. Leithauser D. Rational principles of early ambulation. J Int Coll Surg 1949;12(3):368–374. [PubMed] [Google Scholar]
- 56. Leithauser D. Confinement to bed for only twenty-four hours after operation: a means of preventing pulmonary and circulatory complications and of shortening the period of convalescence. Arch Surg 1943;47(2):203–215. [Google Scholar]
- 57. Leithauser D, Saraf L, Smyka S, et al. Prevention of embolic complications from venous thrombosis after surgery: Standardized regimen of early ambulation. JAMA 1951;147(4):300–303. [DOI] [PubMed] [Google Scholar]
- 58. Sandler H, Vernikos J.. Inactivity: physiological effects. Academic Press: Orlando; 1986. [Google Scholar]
- 59. Dock W. The evil sequelae of complete bed rest. JAMA 1944;125(16):1083-1085. [Google Scholar]
- 60. Asher RAJ. The dangers of going to bed. BMJl 1947;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Browse NL. The physiology and pathology of bed rest. Thomas: Springfield, IL.; 1965. [Google Scholar]
- 62. Allen C, Glasziou P, Mar CD. Bed rest: a potentially harmful treatment needing more careful evaluation. Lancet 1999;354(9186):1229–1233; doi: 10.1016/s0140-6736(98)10063-6 [DOI] [PubMed] [Google Scholar]
- 63. De Kruijk JR, Twijnstra A, Meerhoff S, et al. Management of mild traumatic brain injury: lack of consensus in Europe. Brain Inj 2001;15(2):117–123; doi: 10.1080/026990501458353 [DOI] [PubMed] [Google Scholar]
- 64. Symonds CP, Russell WR. Accidental head injuries—prognosis in service patients. Lancet 1943;1:7–10. [Google Scholar]
- 65. Müller R, Naumann B. Early ambulation and psychotherapy for treatment of closed head injury. Arch Neurol Psychiatry 1956;76(6):597–607; doi: 10.1001/archneurpsyc.1956.02330300027004 [DOI] [PubMed] [Google Scholar]
- 66. Andreassen J, Bach-Nielsen P, Heckscher H, et al. Reassurance and short period of bed rest in the treatment of concussion; follow-up and comparison with results in other series treated by prolonged bed rest. Acta Med Scand 1957;158(3-4):239–248; doi: 10.1111/j.0954-6820.1957.tb15488.x [DOI] [PubMed] [Google Scholar]
- 67. Minderhoud J, Boelens M, Huizenga J, et al. Treatment of minor head injuries. Clin Neurol Neurosurg 1980;82(2):127–140; doi: 10.1016/0303-8467(80)90007-4 [DOI] [PubMed] [Google Scholar]
- 68. Kelly JP, Nichols JS, Filley CM, et al. Concussion in sports: guidelines for the prevention of catastrophic outcome. JAMA 1991;266(20):2867–2869; doi: 10.1001/jama.266.20.2867 [DOI] [PubMed] [Google Scholar]
- 69. Cantu RC. Second-impact syndrome. Clin Sports Med 1998;17(1):37–44; doi: 10.1016/s0278-5919(05)70059-4 [DOI] [PubMed] [Google Scholar]
- 70. Moser RS. The growing public health concern of sports concussion: the new psychology practice frontier. Prof Psychology Res Pract 2007;38(6):699. [Google Scholar]
- 71. McCrea M, Guskiewicz K, Randolph C, et al. Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery 2009;65(5):876–882; doi: 10.1227/01.Neu.0000350155.89800.00 [DOI] [PubMed] [Google Scholar]
- 72. Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Curr Sports Med Rep 2013;12(6):370–376; doi: 10.1249/JSR.000000000000000 [DOI] [PubMed] [Google Scholar]
- 73. Balasundaram AP, Sullivan JS, Schneiders AG, et al. Symptom response following acute bouts of exercise in concussed and non-concussed individuals—a systematic narrative review. Phys Ther Sport 2013;14(4):253–258; doi: 10.1016/j.ptsp.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 74. Silverberg ND, Iverson GL, McCrea M, et al. Activity-related symptom exacerbations after pediatric concussion. JAMA Pediatr 2016;170(10):946–953; doi: 10.1001/jamapediatrics.2016.1187 [DOI] [PubMed] [Google Scholar]
- 75. Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Training 2001;36(3):228. [PMC free article] [PubMed] [Google Scholar]
- 76. Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery 2014; 75 Suppl 4(0 4):S24–S33; doi: 10.1227/NEU.0000000000000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ip EY, Zanier ER, Moore AH, et al. Metabolic, neurochemical, and histologic responses to vibrissa motor cortex stimulation after traumatic brain injury. J Cereb Blood Flow Metab 2003;23(8):900–910; doi: 10.1097/01.WCB.0000076702.71231.F2 [DOI] [PubMed] [Google Scholar]
- 78. Maugans TA, Farley C, Altaye M, et al. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics 2012;129(1):28–37; doi: 10.1542/peds.2011-2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Haykowsky MJ, Eves ND, Warburton DE, et al. Resistance exercise, the Valsalva maneuver, and cerebrovascular transmural pressure. Med Sci Sports Exerc 2003;35(1):65–68; doi: 10.1097/00005768-200301000-00011 [DOI] [PubMed] [Google Scholar]
- 80. Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res 2004;1016(2):154–162; doi: 10.1016/j.brainres.2004.04.079 [DOI] [PubMed] [Google Scholar]
- 81. Griesbach GS, Hovda DA, Molteni R, et al. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience 2004;125(1):129–139; doi: 10.1016/j.neuroscience.2004.01.030 [DOI] [PubMed] [Google Scholar]
- 82. Griesbach GS, Tio DL, Vincelli J, et al. Differential effects of voluntary and forced exercise on stress responses after traumatic brain injury. J Neurotrauma 2012;29(7):1426–1433; doi: 10.1089/neu.2011.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ko IG, Kim SE, Hwang L, et al. Late starting treadmill exercise improves spatial leaning ability through suppressing CREP/BDNF/TrkB signaling pathway following traumatic brain injury in rats. J Exerc Rehabil 2018;14(3):327–334; doi: 10.12965/jer.1836248.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Karelina K, Schneiderman K, Shah S, et al. Moderate intensity treadmill exercise increases survival of newborn hippocampal neurons and improves neurobehavioral outcomes after traumatic brain injury. J Neurotrauma 2021;38(13):1858–1869; doi: 10.1089/neu.2020.7389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Piao CS, Stoica BA, Wu J, et al. Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiol Dis 2013;54:252–263; doi: 10.1016/j.nbd.2012.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shen X, Li A, Zhang Y, et al. The effect of different intensities of treadmill exercise on cognitive function deficit following a severe controlled cortical impact in rats. Int J Mol Sci 2013;14(11):21598–21612; doi: 10.3390/ijms141121598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Griesbach GS, Gomez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma 2007;24(7):1161–1171; doi: 10.1089/neu.2006.0255 [DOI] [PubMed] [Google Scholar]
- 88. Relander M, Troupp H, Af Björkesten G. Controlled trial of treatment for cerebral concussion. BMJ 1972;4(5843):777–779; doi: 10.1136/bmj.4.5843.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. de Kruijk JR, Leffers P, Meerhoff S, et al. Effectiveness of bed rest after mild traumatic brain injury: a randomised trial of no versus six days of bed rest. J Neurol Neurosurg Psychiatry 2002;73(2):167–172; doi: 10.1136/jnnp.73.2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Thomas DG, Apps JN, Hoffmann RG, et al. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics 2015;135(2):213–223; doi: 10.1542/peds.2014-0966 [DOI] [PubMed] [Google Scholar]
- 91. Buckley TA, Munkasy BA, Clouse BP. Acute cognitive and physical rest may not improve concussion recovery time. J Head Trauma Rehabil 2016;31(4):233–241; doi: 10.1097/htr.0000000000000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cassilo D, Sanderson J. From social isolation to becoming an advocate: exploring athletes' grief discourse about lived concussion experiences in online forums. Commun Sport 2019;7(5):678–696, doi: 10.1177/2167479518790039 [DOI] [Google Scholar]
- 93. Smorawiński J, Nazar K, Kaciuba-Uscilko H, et al. Effects of 3-day bed rest on physiological responses to graded exercise in athletes and sedentary men. J Appl Physiol (1985) 2001;91(1):249–257; doi: 10.1152/jappl.2001.91.1.249 [DOI] [PubMed] [Google Scholar]
- 94. Purkayastha S, Stokes M, Bell KR. Autonomic nervous system dysfunction in mild traumatic brain injury: a review of related pathophysiology and symptoms. Brain Inj 2019;33(9):1129–1136; doi: 10.1080/02699052.2019.1631488 [DOI] [PubMed] [Google Scholar]
- 95. Albalawi T, Hamner JW, Lapointe M, et al. The relationship between cerebral vasoreactivity and post-concussive symptom severity. J Neurotrauma 2017;34(19):2700–2705; doi: 10.1089/neu.2017.5060 [DOI] [PubMed] [Google Scholar]
- 96. Esterov D, Greenwald BD. Autonomic dysfunction after mild traumatic brain injury. Brain Sci 2017;7(8); doi: 10.3390/brainsci7080100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. DiFazio M, Silverberg ND, Kirkwood MW, et al. Prolonged activity restriction after concussion: are we worsening outcomes? Clin Pediatr (Phila) 2016;55(5):443–451; doi: 10.1177/0009922815589914 [DOI] [PubMed] [Google Scholar]
- 98. Willer BS, Haider MN, Bezherano I, et al. Comparison of rest to aerobic exercise and placebo-like treatment of acute sport-related concussion in male and female adolescents. Arch Phys Med Rehabil 2019;100(12):2267–2275; doi: 10.1016/j.apmr.2019.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Leddy JJ, Haider MN, Ellis MJ, et al. Early subthreshold aerobic exercise for sport-related concussion: a randomized clinical trial. JAMA Pediatr 2019;173(4):319–325; doi: 10.1001/jamapediatrics.2018.4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Silverberg ND, Otamendi T, Panenka WJ, et al. De-implementing prolonged rest advice for concussion in primary care settings: a pilot stepped wedge cluster randomized trial. J Head Trauma Rehabil 2021;36(2):79–86; doi: 10.1097/HTR.0000000000000609 [DOI] [PubMed] [Google Scholar]
- 101. Lumba-Brown A, Yeates KO, Sarmiento K, et al. Centers for Disease Control and Prevention Guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr 2018;172(11); ARTN e182853; doi: 10.1001/jamapediatrics.2018.2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Purcell L. What are the most appropriate return-to-play guidelines for concussed child athletes? Br J Sports Med 2009;43(Suppl 1):i51–i55; doi: 10.1136/bjsm.2009.058214 [DOI] [PubMed] [Google Scholar]
- 103. Field M, Collins MW, Lovell MR, et al. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr 2003;142(5):546–553; doi: 10.1067/mpd.2003.190 [DOI] [PubMed] [Google Scholar]
- 104. McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 2013;47(5):250–258; doi: 10.1136/bjsports-2013-092313 [DOI] [PubMed] [Google Scholar]
- 105. Zemek R, Eady K, Moreau K, et al. Knowledge of paediatric concussion among front-line primary care providers. Paed Child Health 2014;19(9):474–479; doi: 10.1093/pch/19.9.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Arbogast KB, McGinley AD, Master CL, et al. Cognitive rest and school-based recommendations following pediatric concussion: the need for primary care support tools. Clin Pediatr 2013;52(5):397–402; doi: 10.1177/0009922813478160 [DOI] [PubMed] [Google Scholar]
- 107. Moor HM, Eisenhauer RC, Killian KD, et al. The relationship between adherence behaviors and recovery time in adolescents after a sports-related concussion: an observational study. Int J Sports Phys Ther 2015;10(2):225–233. [PMC free article] [PubMed] [Google Scholar]
- 108. Gagnon I, Swaine B, Forget R. Using activity diaries to measure children's and adolescents' compliance with activity restrictions after mild traumatic brain injury. J Head Trauma Rehabil 2009;24(5):355–362; doi: 10.1097/HTR.0b013e3181b97a4e [DOI] [PubMed] [Google Scholar]
- 109. Silverberg ND, Otamendi T. Advice to rest for more than 2 days after mild traumatic brain injury is associated with delayed return to productivity: a case-control study. Front Neurol 2019;10(362; doi: 10.3389/fneur.2019.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Macnow T, Curran T, Tolliday C, et al. Effect of screen time on recovery from concussion: a randomized clinical trial. JAMA Pediatr 2021;175(11):1124–1131; doi: 10.1001/jamapediatrics.2021.2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.