Extended Data Fig. 5. Validation of PTR-25/PTCHD1 as causal gene mutated in tgMOR; bgg10 animals that results in opioid hypersensitivity.
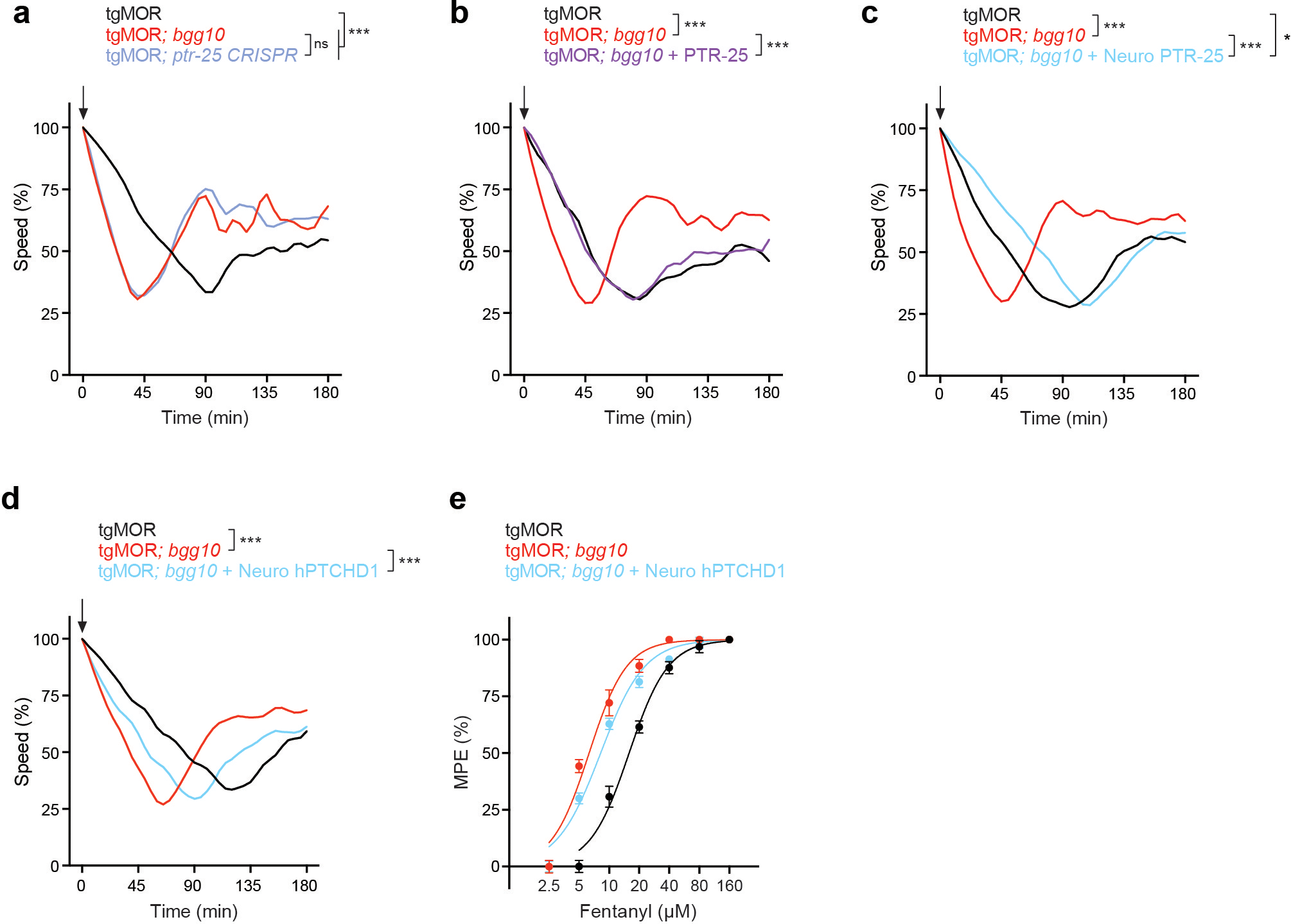
a, Time course of fentanyl application for indicated genotypes. Arrow indicates application of fentanyl (10μM). TgMOR; ptr-25 CRISPR animals phenocopied hypersensitivity in tgMOR; bgg10 mutants. b, Native rescue with PTR-25 restored opioid sensitivity of tgMOR; bgg10 animals. c, Pan-neuronal rescue (rab-3 promoter) with PTR-25 reversed opioid sensitivity of tgMOR; bgg10 mutants. d, Time course of fentanyl application (5μM, arrow) for indicated genotypes. Note transgenic expression of human PTCHD1 using the native ptr-25 promoter rescues opioid hypersensitivity of tgMOR; bgg10 animals. e, Dose-response curves with fentanyl showing transgenic human PTCHD1 decreased opioid sensitivity of tgMOR; bgg10 animals. For a-d, plotted is mean data for n=12 wells (4–5 animals/well) for all genotypes. For e, n=3 independent experiments with each experiment derived from 4 wells (4–5 animals/well) per dose for all genotypes. Data points are means ± SD. For a-d, data were analyzed by two-way ANOVA with Bonferroni’s post hoc test. ***p<0.001, * p<0.05
