FIG 7.
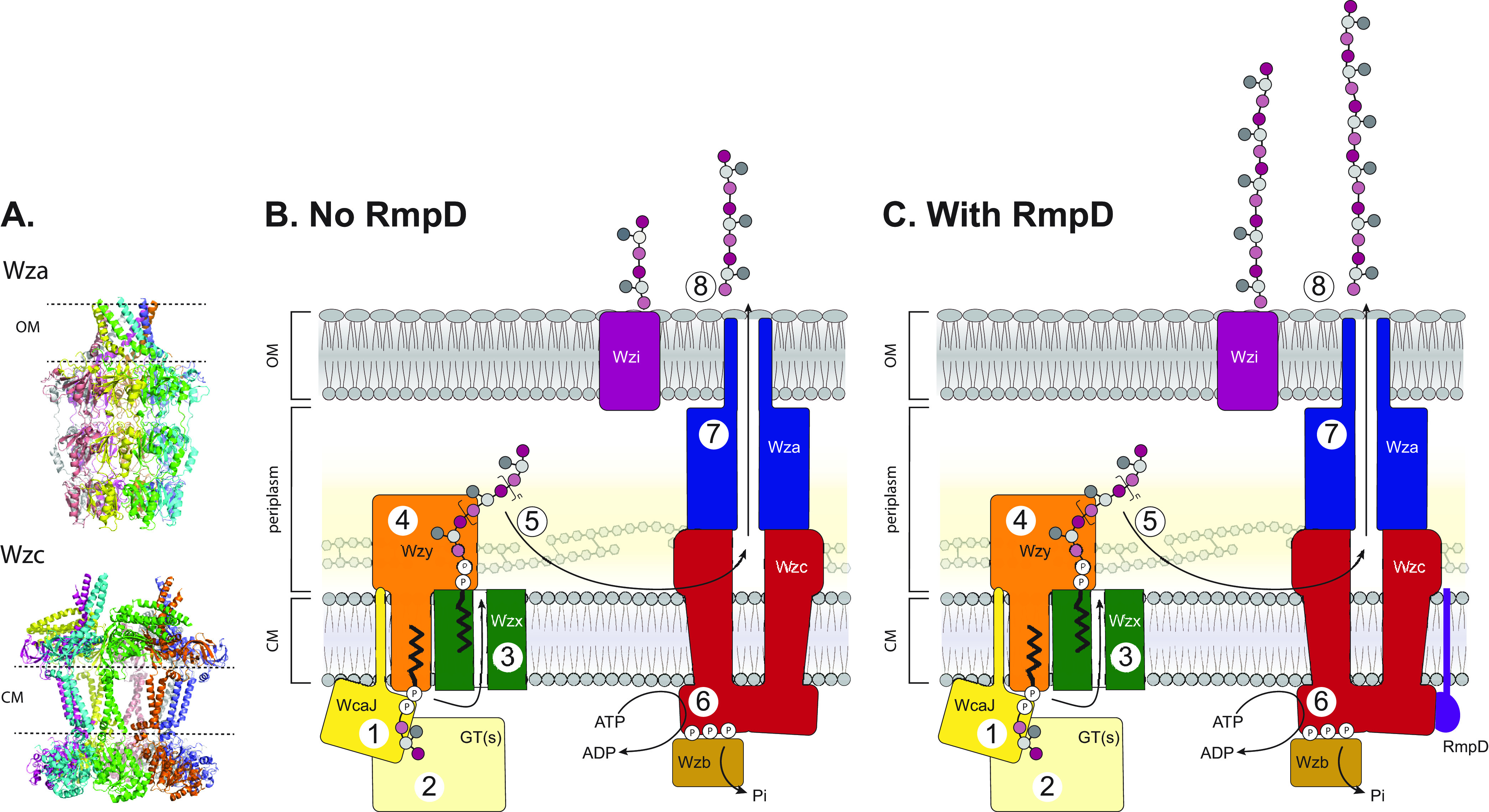
Cellular locations and known/proposed functions of the proteins required for biosynthesis and export of K. pneumoniae CPS. (A) The solved structures of Wza and Wzc multimers. (B) Synthesis of CPS repeat units is initiated on an undecaprenol phosphate acceptor by a phosphoglycosyltransferase (WcaJ, 1) and completed by the activities of serotype-specific glycosyltransferases (GTs; 2). The lipid-linked repeat units are exported via Wzx, a member of the MOPS transporter family (3) and polymerized by a Wzy homolog (4). This is followed by translocation involving a complex comprising octamers of the PCP-2a (Wzc) and OPX (Wza) proteins (5–7). Wzc plays dual roles in polymerization and translocation driven by its reversible phosphorylation (6). This likely requires direct interaction between Wzy and Wzc proteins based on data for Wzy-Wzz interactions in other systems which place Wzy within the Wzz lumen (69). Since the details and relative locations of synthesis and export-translocation proteins are not yet established by direct experiments for CPS biosynthesis, they are not inferred in the model shown here. While the capsular polymers are closely associated with the cell surface, the underlying processes for the association are not fully understood. The lectin-like activity of Wzi is involved but is likely not sufficient, and a substantial amount of the polysaccharide product is released from the cell. (C) In the presence of RmpD, CPS chains are elongated, likely through an interaction with Wzc. The location and nature of this interaction is not known. This figure is a modified version of a model published previously (26).
