Figure 1.
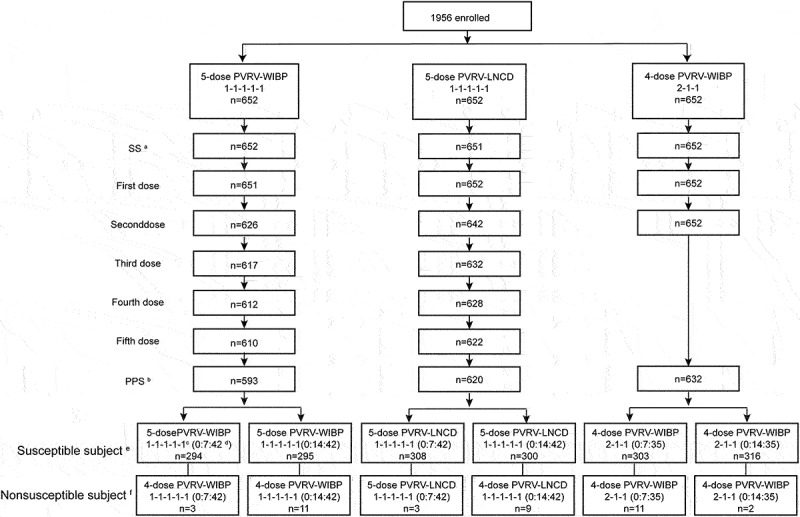
Flow diagram of the stage 2.(a) SS, safety analysis set. All individuals received the first vaccination after randomization. (b) PPS, per-protocol analysis set. The subjects with better compliance and who completed the vaccination schedule with the safety and immunogenicity indexes were recruited. Individuals excluded from the PPS had incomplete inoculation/no pre-immunization test results or violated/deviated from the protocol. (c) The 1-1-1-1-1 and 2-1-1 regimens indicate the vaccination schedule. (d) The 0:7:42 and 0:14:42 indicate the blood collection on days 0, 7, and 42 or days 0, 14, and 42 in the 5-dose PVRV-WIBP or PVRV-LNCD groups. The 0:7:35 and 0:14:35 indicate blood collection on days 0, 7, and 35 or days 0, 14, and 35 in the 4-dose PVRV-WIBP group. (e) In susceptible subjects, the pre-immunization RFFIT antibody titer of the individual is <0.5 IU/mL. (f) Nonsusceptible subjects, pre-immunization RFFIT antibody titer of the individual is ≥0.5 IU/mL.
