Figure 1.
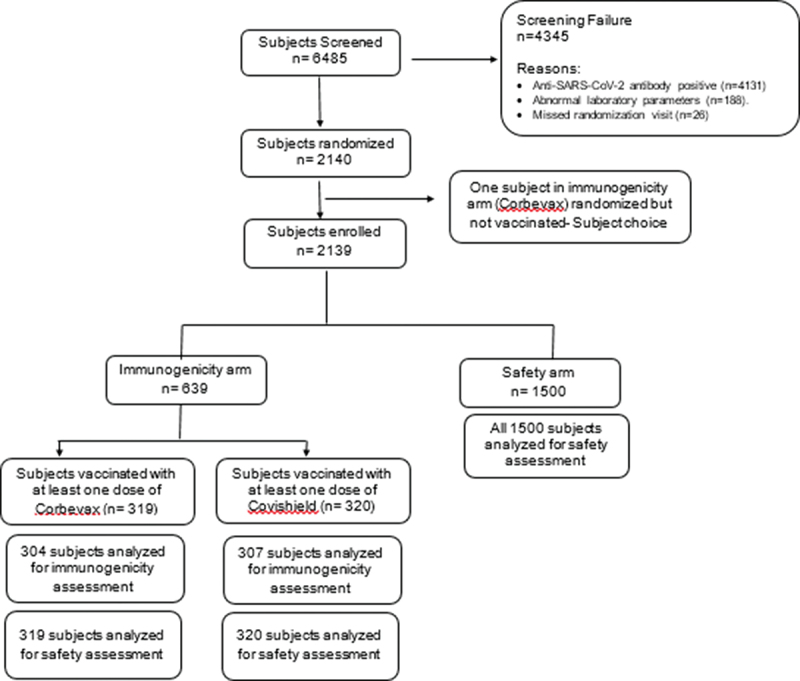
Subject disposition (consort diagram).
A total of 6485 subjects were screened, and 2140 subjects were randomized to either immunogenicity arm or safety arm. Anti-SARS-CoV-2 antibody positive status and abnormal laboratory parameters were the reasons for screen failure of the subjects. One subject randomized in the immunogenicity arm refused to receive the study vaccine. So, in total, 2139 subjects received at least one dose of the study vaccine. In the immunogenicity arm (n = 639), subjects received either CORBEVAX™ vaccine (n = 319) or COVISHIELD™ vaccine (n = 320). All subjects were analysed for safety assessment in this arm. Immunogenicity assessment was performed in n = 304 and n = 307 subjects in CORBEVAX™ or COVISHIELD™ vaccinated groups, respectively. All 1500 subjects in safety arm were included for safety analysis.
n, number; RBD; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
